Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
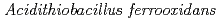
Kanao, Tadayoshi*; Kosaka, Megumi*; Yoshida, Kyoya*; Nakayama, Hisayuki*; Tamada, Taro; Kuroki, Ryota; Yamada, Hidenori; Takada, Jun*; Kamimura, Kazuo*
Acta Crystallographica Section F, 69(6), p.692 - 694, 2013/06
Times Cited Count:10 Percentile:58.18(Biochemical Research Methods)Tetrathionate hydrolase (4THase) from the iron- and sulfur-oxidizing bacterium 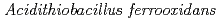 catalyses the disproportionate hydrolysis of tetrathionate to elemental sulfur, thiosulfate and sulfate. The gene encoding 4THase (
catalyses the disproportionate hydrolysis of tetrathionate to elemental sulfur, thiosulfate and sulfate. The gene encoding 4THase (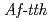 ) was expressed as inclusion bodies in recombinant
) was expressed as inclusion bodies in recombinant  . Recombinant
. Recombinant 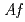 -Tth was activated by refolding under acidic conditions and was then purified to homogeneity. The recombinant protein was crystallized in 20 m
-Tth was activated by refolding under acidic conditions and was then purified to homogeneity. The recombinant protein was crystallized in 20 m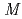 glycine buffer pH 10 containing 50 m
glycine buffer pH 10 containing 50 m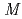 sodium chloride and 33%(
sodium chloride and 33%(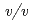 ) PEG 1000 using the hanging-drop vapour-diffusion method. The crystal was a hexagonal cylinder with dimensions of 0.2
) PEG 1000 using the hanging-drop vapour-diffusion method. The crystal was a hexagonal cylinder with dimensions of 0.2  0.05
0.05  0.05 mm. X-ray crystallographic analysis showed that the crystal diffracted to 2.15
0.05 mm. X-ray crystallographic analysis showed that the crystal diffracted to 2.15  resolution and belongs to space group
resolution and belongs to space group  3
3 or
or  3
3 , with unit-cell parameters
, with unit-cell parameters  =
=  = 92.1,
= 92.1,  = 232.6
= 232.6  .
.
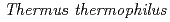 HB8
HB8Okazaki, Nobuo; Adachi, Motoyasu; Tamada, Taro; Kurihara, Kazuo; Oga, Takushi*; Kamiya, Nobuo*; Kuramitsu, Seiki*; Kuroki, Ryota
Acta Crystallographica Section F, 68(1), p.49 - 52, 2012/01
Times Cited Count:2 Percentile:31.75(Biochemical Research Methods)Fukuda, Yota*; Tamada, Taro; Takami, Hideto*; Suzuki, Shinichiro*; Inoue, Tsuyoshi*; Nojiri, Masaki
Acta Crystallographica Section F, 67(6), p.692 - 695, 2011/06
Times Cited Count:10 Percentile:66.87(Biochemical Research Methods)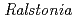 sp. A-471
sp. A-471Okazaki, Nobuo; Arimori, Takao; Nakazawa, Masami*; Miyatake, Kazutaka*; Ueda, Mitsuhiro*; Tamada, Taro
Acta Crystallographica Section F, 67(4), p.494 - 497, 2011/04
Times Cited Count:3 Percentile:40.30(Biochemical Research Methods)
Yamada, Mitsugu; Sato, Katsuya; Narumi, Issei
Acta Crystallographica Section F, 66(12), p.1614 - 1616, 2010/12
Times Cited Count:1 Percentile:15.54(Biochemical Research Methods)DNA damage response A protein (DdrA) from  has been suggested to be involved in DNA repair processes through binding to 3' ends of single-stranded DNA, thereby protecting the ends from nuclease digestion. In this study, a recombinant C-terminal truncated form of
has been suggested to be involved in DNA repair processes through binding to 3' ends of single-stranded DNA, thereby protecting the ends from nuclease digestion. In this study, a recombinant C-terminal truncated form of 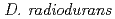 DdrA (DdrA157), comprising of the first 157 residues of DdrA, was expressed in
DdrA (DdrA157), comprising of the first 157 residues of DdrA, was expressed in 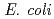 , purified and crystallized. Single crystals of DdrA157 were obtained by a hanging drop method at 293 K. The crystal belonged to the monoclinic space group
, purified and crystallized. Single crystals of DdrA157 were obtained by a hanging drop method at 293 K. The crystal belonged to the monoclinic space group  2
2 , with unit-cell parameters
, with unit-cell parameters  = 46.31,
= 46.31,  = 180.26,
= 180.26,  = 114.17
= 114.17  ,
, 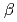 = 90.02
= 90.02 . The crystal was expected to contain fourteen molecules in the asymmetric unit. Diffraction data were collected to 2.35
. The crystal was expected to contain fourteen molecules in the asymmetric unit. Diffraction data were collected to 2.35  resolution at beamline BL-5 of the Photon Factory and initial phase determinations were attempted by a molecular-replacement method using the human Rad52 structure.
resolution at beamline BL-5 of the Photon Factory and initial phase determinations were attempted by a molecular-replacement method using the human Rad52 structure.
Matsumura, Hiroyoshi*; Adachi, Motoyasu; Sugiyama, Shigeru*; Okada, Shino*; Yamakami, Megumi*; Tamada, Taro; Hidaka, Koshi*; Hayashi, Yoshio*; Kimura, Toru*; Kiso, Yoshiaki*; et al.
Acta Crystallographica Section F, 64(11), p.1003 - 1006, 2008/11
Times Cited Count:17 Percentile:77.21(Biochemical Research Methods)This paper reports the crystallization and preliminary neutron diffraction measurements of HIV-1 protease, a potential target for anti-HIV therapy, complexed with an inhibitor (KNI-272). The aim of this neutron diffraction study is to obtain structural information about the H atoms and to determine the protonation states of the residues within the active site. The crystal was grown to a size of 1.4 mm by repeated macroseeding and a slow-cooling method using a two-liquid system. Neutron diffraction data were collected at room temperature using a BIX-4 diffractometer at the JRR-3 research reactor of the Japan Atomic Energy Agency (JAEA). The data set was integrated and scaled to 2.3
by repeated macroseeding and a slow-cooling method using a two-liquid system. Neutron diffraction data were collected at room temperature using a BIX-4 diffractometer at the JRR-3 research reactor of the Japan Atomic Energy Agency (JAEA). The data set was integrated and scaled to 2.3  resolution in space group P2(1)2(1)2, with unit-cell parameters a = 59.5, b = 87.4, c = 46.8
resolution in space group P2(1)2(1)2, with unit-cell parameters a = 59.5, b = 87.4, c = 46.8  .
.
Taguchi, Chiho; Taura, Futoshi*; Tamada, Taro; Shoyama, Yoshinari; Shoyama, Yukihiro*; Tanaka, Hiroyuki*; Kuroki, Ryota; Morimoto, Satoshi*
Acta Crystallographica Section F, 64(3), p.217 - 220, 2008/03
Times Cited Count:3 Percentile:35.30(Biochemical Research Methods)Kinoshita, Takayoshi*; Tamada, Taro; Imai, Keisuke*; Kurihara, Kazuo; Ohara, Takashi; Kuroki, Ryota
Acta Crystallographica Section F, 63(4), p.315 - 317, 2007/04
Times Cited Count:8 Percentile:65.76(Biochemical Research Methods)Porcine pancreatic elastase (PPE) resembles the attractive drug target leukocyte elastase, which has been implicated in a number of inflammatory disorders. In order to investigate the structural characteristics of the covalent inhibitor bound to PPE, including hydrogen and hydration of water, a single crystal of PPE for neutron diffraction study was grown in D O containing 0.2 M sodium sulfate (pD=5.0) using the sitting-drop vapor diffusion method. The crystal was grown to a size of 1.6 mm
O containing 0.2 M sodium sulfate (pD=5.0) using the sitting-drop vapor diffusion method. The crystal was grown to a size of 1.6 mm by repeated macro-seeding. The neutron diffraction data were collected at room temperature using a BIX-3 diffractometer at the JRR-3 research reactor of the Japan Atomic Energy Agency (JAEA). The data set was integrated and scaled to 2.3
by repeated macro-seeding. The neutron diffraction data were collected at room temperature using a BIX-3 diffractometer at the JRR-3 research reactor of the Japan Atomic Energy Agency (JAEA). The data set was integrated and scaled to 2.3 resolution with space group P212121 and cell dimensions of a=51.2
resolution with space group P212121 and cell dimensions of a=51.2 , b=57.8
, b=57.8 and c=75.6
and c=75.6 .
.
Honjo, Eijiro; Tamada, Taro; Maeda, Yoshitake*; Koshiba, Takumi*; Matsukura, Yasuko*; Okamoto, Tomoyuki*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
Acta Crystallographica Section F, 61(8), p.788 - 790, 2005/08
Times Cited Count:7 Percentile:54.61(Biochemical Research Methods)Granulocyte colony-stimulating factor (GCSF) receptor receives signals for regulating the proliferation and differentiation of the precursor cells of granulocytes. The complex composed of two GCSFs and two GCSF receptors was crystallized. The crystal of the complex was grown in 1.0 M sodium formate and 0.1 M sodium acetate (pH4.6). It belongs to the space group  4
4 2
2 2 (or its enantiomorph
2 (or its enantiomorph  4
4 2
2 2) with unit cell dimensions of
2) with unit cell dimensions of  =
=  = 110.1
= 110.1  ,
,  = 331.8
= 331.8  . However, the diffraction data from the crystal beyond 5
. However, the diffraction data from the crystal beyond 5  resolution could not be collected. Since the heterogeneity of GCSF receptor seems to interrupt growth of good quality crystals, the GCSF receptor was fractionated by achromatography. Crystals of GCSF/fractionated GCSF receptor complex were grown as a new crystal form in 0.2 M ammonium phosphate. The new crystal diffracts beyond 3.0
resolution could not be collected. Since the heterogeneity of GCSF receptor seems to interrupt growth of good quality crystals, the GCSF receptor was fractionated by achromatography. Crystals of GCSF/fractionated GCSF receptor complex were grown as a new crystal form in 0.2 M ammonium phosphate. The new crystal diffracts beyond 3.0  resolution and belongs to space group
resolution and belongs to space group  3
3 21 (or its enantiomorph
21 (or its enantiomorph  3
3 21) with unit-cell parameters
21) with unit-cell parameters  =
=  = 134.8,
= 134.8,  = 105.7
= 105.7  .
.