Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Mo adsorption; Utilizing spray-dried mesoporous alumina for clinical-grade generator development
Mo adsorption; Utilizing spray-dried mesoporous alumina for clinical-grade generator developmentAlowasheeir, A.*; Eguchi, Miharu*; Fujita, Yoshitaka; Tsuchiya, Kunihiko; Wakabayashi, Ryutaro*; Kimura, Tatsuo*; Ariga, Katsuhiko*; Hatano, Kentaro*; Fukumitsu, Nobuyoshi*; Yamauchi, Yusuke*
Bulletin of the Chemical Society of Japan, 97(10), p.uoae099_1 - uoae099_7, 2024/10
Times Cited Count:3 Percentile:37.20(Chemistry, Multidisciplinary)no abstracts in English
Sekine, Yurina; Nankawa, Takuya
Bulletin of the Chemical Society of Japan, 96(10), p.1150 - 1155, 2023/10
Times Cited Count:4 Percentile:16.62(Chemistry, Multidisciplinary)The phase separation of ice crystals and solutes and bound water that occurs during freezing can be used as a reaction field to control a hierarchical structure of hydrogels. Here, we present a study of carboxymethyl cellulose nanofiber (CMCF) hydrogels formed using the solid-quasi liquid phase separation. CMCF hydrogels were formed simply by adding citric acid to frozen CMCF and thawing the mixture. It was found that rearrangement of CMCF structures via hydrogen bonding proceeds in the freeze concentration layer before the ice crystals melt. Under freeze concentration, CMCF and bound water are confined at high concentrations. The crosslinking reaction in such a unique space contributed to the formation of CMCF hydrogel with high mechanical strength. We discuss the gelation behavior and properties of freeze crosslinked CMCF hydrogels and their applications.
Miyagawa, Akihisa*; Hayashi, Naoki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; Nakatani, Kiyoharu*
Bulletin of the Chemical Society of Japan, 96(9), p.1019 - 1025, 2023/09
Times Cited Count:4 Percentile:34.10(Chemistry, Multidisciplinary)In the present study, we have elucidated the mass transfer mechanism of Eu(III) and Sm(III) in the solution with these ions in single nitrilotriacetamide (NTA) extractant-impregnated polymer-coated silica particle. The rate-limiting process of mass transfer was the reaction process of ions with NTA molecules, in which the NO
 ions were not involved, which was consistent with that obtained in single ion distribution system.
ions were not involved, which was consistent with that obtained in single ion distribution system.
Miyagawa, Akihisa*; Hayashi, Naoki*; Kuzure, Yoshiaki*; Takahashi, Takumi*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; et al.
Bulletin of the Chemical Society of Japan, 96(7), p.671 - 676, 2023/07
Times Cited Count:7 Percentile:58.25(Chemistry, Multidisciplinary)We investigated the distribution mechanism of Eu(III) in a single polymer-coated silica particle including nitrilotriacetamide (NTA) extractants known as HONTA and TOD2EHNTA. The present study provides a valuable approach for the evaluation and enhancement of the functionality of "single extractant-impregnated polymer-coated silica particle".
Saito, Shingo*; Haraga, Tomoko; Marumo, Kazuki*; Sato, Yoshiyuki; Nakano, Yuta*; Tasaki-Handa, Yuiko*; Shibukawa, Masami*
Bulletin of the Chemical Society of Japan, 96(3), p.223 - 225, 2023/03
Times Cited Count:3 Percentile:34.10(Chemistry, Multidisciplinary)Highly efficient and effective separation between americium (Am ) and curium ion (Cm
) and curium ion (Cm ) was achieved by two simple electrophoresis-based techniques. Am
) was achieved by two simple electrophoresis-based techniques. Am and Cm
and Cm ions were complexed with fluorophore-modified acyclic hexadentate and octadentate polyaminocarboxylates and then were electrophoretically separated and fluorescently detected in free solution with ternary complexation or in gel medium.
ions were complexed with fluorophore-modified acyclic hexadentate and octadentate polyaminocarboxylates and then were electrophoretically separated and fluorescently detected in free solution with ternary complexation or in gel medium.
Nankawa, Takuya; Sekine, Yurina; Yamada, Teppei*
Bulletin of the Chemical Society of Japan, 95(5), p.825 - 829, 2022/05
Times Cited Count:4 Percentile:29.12(Chemistry, Multidisciplinary)Advances in hazardous metal ion removal are essential for wastewater clean-up to tackle the global water shortage crisis. Here, we report a Pb-selective adsorbent using a Tb oxalate framework (TOF) synthesized by a one-pot hydrothermal method. The TOF has a two-dimensional sheet structure, in which the interlayer space functions as an ion exchangeable site. Sorption tests using a mixed-ion solution containing Pb , Cd
, Cd , Mn
, Mn , Co
, Co , Ni
, Ni , Cu
, Cu , Na
, Na , K
, K , Mg
, Mg , and Ca
, and Ca showed that the TOF has high selectivity for Pb
showed that the TOF has high selectivity for Pb among other metal ions. The saturated adsorption capacity of the TOF for Pb
among other metal ions. The saturated adsorption capacity of the TOF for Pb was 276 mg g
was 276 mg g , which is higher than that of conventional adsorbents. Furthermore, the TOF exhibited reversible Pb
, which is higher than that of conventional adsorbents. Furthermore, the TOF exhibited reversible Pb adsorption/desorption and could be used for at least three cycles. The results showed that TOF has excellent potential as an adsorbent for removing Pb
adsorption/desorption and could be used for at least three cycles. The results showed that TOF has excellent potential as an adsorbent for removing Pb , and because of its reusability, it is also a promising material for wastewater clean-up.
, and because of its reusability, it is also a promising material for wastewater clean-up.
Miyagawa, Akihisa*; Takeuchi, Masayuki; Arai, Tsuyoshi*; Watanabe, So; Sano, Yuichi; Nakatani, Kiyoharu*
Bulletin of the Chemical Society of Japan, 95(4), p.566 - 568, 2022/04
Times Cited Count:3 Percentile:21.39(Chemistry, Multidisciplinary)We demonstrate that pKa of extractant impregnated in a polymer phase varies with the cross-linking degree and the coexistence of other extractants, which induces a change in the hydrophobicity of the polymer phase. The results presented herein will be beneficial for the development of novel solid-extraction adsorbents.
Fujita, Yoshitaka; Niizeki, Tomotake*; Fukumitsu, Nobuyoshi*; Ariga, Katsuhiko*; Yamauchi, Yusuke*; Malgras, V.*; Kaneti, Y. V.*; Liu, C.-H.*; Hatano, Kentaro*; Suematsu, Hisayuki*; et al.
Bulletin of the Chemical Society of Japan, 95(1), p.129 - 137, 2022/01
Times Cited Count:10 Percentile:61.73(Chemistry, Multidisciplinary)In this work, the mechanisms responsible for the adsorption of molybdate ions on alumina are investigated using in-depth surface analyses carried out on alumina specimens immersed in solutions containing different molybdate ions at different pH values. The obtained results reveal that when alumina is immersed in an acidic solution containing molybdate ions, the hydroxyl groups present on the surface are removed to generate positively charged sites, and molybdate ions (MoO
 or AlMo
or AlMo O
O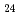 H
H
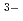 ) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo
) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo O
O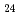 H
H
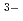 , which is more easily desorbed than MoO
, which is more easily desorbed than MoO
 . Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
. Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
 ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope (
ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope ( Mo/
Mo/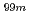 Tc) generators.
Tc) generators.
Benu, D. P.*; Earnshaw, J.*; Ashok, A.*; Tsuchiya, Kunihiko; Saptiama, I.*; Yuliarto, B.*; Suendo, V.*; Mukti, R. R.*; Fukumitsu, Nobuyoshi*; Ariga, Katsuhiko*; et al.
Bulletin of the Chemical Society of Japan, 94(2), p.502 - 507, 2021/02
Times Cited Count:12 Percentile:55.58(Chemistry, Multidisciplinary)no abstracts in English
Imai, Yosuke*; Tokiwa, Yuhei*; Ueno, Shusaku*; Tanida, Hajime; Watanabe, Iwao*; Matsubara, Hiroki*; Takiue, Takanori*; Aratono, Makoto*
Bulletin of the Chemical Society of Japan, 91(10), p.1487 - 1494, 2018/10
Times Cited Count:2 Percentile:7.15(Chemistry, Multidisciplinary)Competitive binding of binary mixed counterions to the headgroups of adsorbed surfactant films has been investigated at solution surfaces by total reflection X-ray absorption fine structure (XAFS) spectroscopy. The obtained extended XAFS 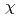 spectra for bromide counterions are linear combinations of the spectra of fully hydrated bromide ions (free Br) and partially dehydrated bromide ions bound to the headgroups of the surfactant ions (bound Br). From the fraction of bound Br in counterion mixed systems, two series of the relative strengths of counterion binding are proposed for the trimethylammonium (TA
spectra for bromide counterions are linear combinations of the spectra of fully hydrated bromide ions (free Br) and partially dehydrated bromide ions bound to the headgroups of the surfactant ions (bound Br). From the fraction of bound Br in counterion mixed systems, two series of the relative strengths of counterion binding are proposed for the trimethylammonium (TA ) and 3-methylimidazolium (MIM
) and 3-methylimidazolium (MIM ) headgroups: (a) TA-SO
) headgroups: (a) TA-SO
 TA-Cl
TA-Cl  TA-Br
TA-Br  TA-BF
TA-BF and (b) MIM-Br
and (b) MIM-Br  TA-Br
TA-Br  TA-BF
TA-BF
 MIM-BF
MIM-BF . For the TA headgroup, matching the hydration of the headgroups and counterions gives series (a) according to Collins' law, which states that the tendency of contact ion pair formation becomes larger when the absolute values of the hydration enthalpies of the ions match. For the MIM headgroup, the number of binding sites of hydrogen bonds between the MIM headgroup and counterion is essential, which leads to series (b) because of competition between the counterion and water for interaction with the MIM headgroup.
. For the TA headgroup, matching the hydration of the headgroups and counterions gives series (a) according to Collins' law, which states that the tendency of contact ion pair formation becomes larger when the absolute values of the hydration enthalpies of the ions match. For the MIM headgroup, the number of binding sites of hydrogen bonds between the MIM headgroup and counterion is essential, which leads to series (b) because of competition between the counterion and water for interaction with the MIM headgroup.
Aoyagi, Noboru; Palladino, G.*; Nagasaki, Shinya*; Kimura, Takaumi
Bulletin of the Chemical Society of Japan, 91(6), p.882 - 890, 2018/06
Times Cited Count:3 Percentile:10.63(Chemistry, Multidisciplinary)The speciation and coordination geometries of M(III)-citrate complexes in aqueous solutions, where M denotes Eu, Tb, Lu, or Cm, are studied using potentiometric titration, nuclear magnetic resonance spectroscopies. Their photophysical properties are also characterized by time-resolved fluorescence spectra. The formation constants of mononuclear, dinuclear, and trinuclear Lu-citrate species were determined by potentiometric titration in 3.00 M NaClO aqueous media. Terminal carboxylic conformation in trinuclear complexes comprised both the five- and six-membered rings at different exchanging rates. Hydration states evaluated for Eu
aqueous media. Terminal carboxylic conformation in trinuclear complexes comprised both the five- and six-membered rings at different exchanging rates. Hydration states evaluated for Eu ions are the chemical formula of [Eu(Cit)
ions are the chemical formula of [Eu(Cit) (H
(H O)
O)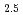 ]
]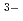 and [Eu
and [Eu (OH)
(OH) (Cit)
(Cit) (H
(H O)
O)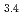 ]
]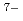 . These complexes in aqueous solution have geometrical similarity to the crystal structures in the literature. Furthermore, the entity of the hetero-trinuclear complex induces the intramolecular energy transfer from Tb
. These complexes in aqueous solution have geometrical similarity to the crystal structures in the literature. Furthermore, the entity of the hetero-trinuclear complex induces the intramolecular energy transfer from Tb to Eu
to Eu . The incorporation of Cm
. The incorporation of Cm into these homo/hetero-trinuclear citrate complexes proved to be a successful trial to probe the formation of actinide polymer at a trace level.
into these homo/hetero-trinuclear citrate complexes proved to be a successful trial to probe the formation of actinide polymer at a trace level.
Saptiama, I.*; Kaneti, Y. V.*; Oveisi, H.*; Suzuki, Yoshitaka; Tsuchiya, Kunihiko; Takai, Kimiko*; Sakae, Takeji*; Pradhan, S.*; Hossain, M. S. A.*; Fukumitsu, Nobuyoshi*; et al.
Bulletin of the Chemical Society of Japan, 91(2), p.195 - 200, 2018/02
Times Cited Count:49 Percentile:80.94(Chemistry, Multidisciplinary)no abstracts in English
Saptiama, I.*; Kaneti, Y. V.*; Suzuki, Yumi*; Suzuki, Yoshitaka; Tsuchiya, Kunihiko; Sakae, Takeji*; Takai, Kimiko*; Fukumitsu, Nobuyoshi*; Alothman, Z. A.*; Hossain, M. S. A.*; et al.
Bulletin of the Chemical Society of Japan, 90(10), p.1174 - 1179, 2017/10
Times Cited Count:45 Percentile:77.14(Chemistry, Multidisciplinary)no abstracts in English
Toyomori, Yuka*; Tsuji, Satoru*; Mitsuda, Shinobu*; Okayama, Yoichi*; Ashida, Shiomi*; Mori, Atsunori*; Kobayashi, Toru; Miyazaki, Yuji; Yaita, Tsuyoshi; Arae, Sachie*; et al.
Bulletin of the Chemical Society of Japan, 89(12), p.1480 - 1486, 2016/09
Times Cited Count:9 Percentile:29.91(Chemistry, Multidisciplinary)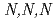 -trimethylglycine
-trimethylglycineSuzuki, Tomoya; Morita, Keisuke; Sasaki, Yuji; Matsumura, Tatsuro
Bulletin of the Chemical Society of Japan, 89(5), p.608 - 616, 2016/05
Times Cited Count:5 Percentile:18.04(Chemistry, Multidisciplinary)In a previous study, we found that AMP03 adsorbs Rh(III) from the HNO solution with pH = 1.7 - 2.2. In this study, to understand the Rh(III) adsorption properties of AMP03 and clarify conditions for efficient recovery, we investigated the adsorption behaviors of Rh(III) from the HNO
solution with pH = 1.7 - 2.2. In this study, to understand the Rh(III) adsorption properties of AMP03 and clarify conditions for efficient recovery, we investigated the adsorption behaviors of Rh(III) from the HNO solution. As a result, it was found that Rh(III) is effectively adsorbed from aqueous solution containing low H
solution. As a result, it was found that Rh(III) is effectively adsorbed from aqueous solution containing low H and high NO
and high NO
 concentration. Considering the adsorption mechanism of Rh(III), we found that Rh
concentration. Considering the adsorption mechanism of Rh(III), we found that Rh in aqueous solution is adsorbed with two betaine groups in AMP03 and three NO
in aqueous solution is adsorbed with two betaine groups in AMP03 and three NO
 .
.
Ninomiya, Kazuhiko; Nagatomo, Takashi*; Kubo, Kenya*; Ito, Takashi; Higemoto, Wataru; Kita, Makoto*; Shinohara, Atsushi*; Strasser, P.*; Kawamura, Naritoshi*; Shimomura, Koichiro*; et al.
Bulletin of the Chemical Society of Japan, 85(2), p.228 - 230, 2012/02
Times Cited Count:29 Percentile:61.01(Chemistry, Multidisciplinary)Elemental analysis of bulk materials can be performed by detecting the high-energy X-rays emitted from muonic atoms. Muon irradiation of standard bronze samples was performed to determine the muon capture probabilities for the elemental components from muonic X-ray spectra. Nondestructive elemental analysis of an ancient Chinese coin was also performed.
Ishii, Yasuo; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Li, Z.*; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; Haba, Hiromitsu*; et al.
Bulletin of the Chemical Society of Japan, 84(9), p.903 - 911, 2011/09
Times Cited Count:18 Percentile:49.65(Chemistry, Multidisciplinary)The cation-exchange behavior of element 104, rutherfordium (Rf), was investigated together with its lighter group-4 homologues Zr and Hf, and the tetravalent pseudo-homologue Th in HF/HNO mixed solution. The results demonstrate that distribution coefficients (
mixed solution. The results demonstrate that distribution coefficients (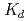 ) of Rf in HF/0.10 M HNO
) of Rf in HF/0.10 M HNO decrease with increasing concentration of the fluoride ion [F
decrease with increasing concentration of the fluoride ion [F ], indicating the consecutive formation of fluorido complexes of Rf. We also measured the
], indicating the consecutive formation of fluorido complexes of Rf. We also measured the 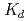 values of Rf and the homologues as a function of the hydrogen ion concentration [H
values of Rf and the homologues as a function of the hydrogen ion concentration [H ]. The log
]. The log 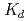 values decrease linearly with an increase of log [H
values decrease linearly with an increase of log [H ] with slopes between -2.1 and -2.5. This indicates that these elements are likely to form the same chemical compounds: mixture of [MF]
] with slopes between -2.1 and -2.5. This indicates that these elements are likely to form the same chemical compounds: mixture of [MF] and [MF
and [MF ]
] (M = Rf, Zr, Hf and Th) in the studied solution. It is also ascertained that sequence in the fluoride complex formation is Zr
(M = Rf, Zr, Hf and Th) in the studied solution. It is also ascertained that sequence in the fluoride complex formation is Zr  Hf
Hf  Rf
Rf  Th.
Th.
Haba, Hiromitsu*; Akiyama, Kazuhiko*; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Yaita, Tsuyoshi; Hirata, Masaru; Sueki, Keisuke*; Nagame, Yuichiro
Bulletin of the Chemical Society of Japan, 82(6), p.698 - 703, 2009/06
Times Cited Count:10 Percentile:39.12(Chemistry, Multidisciplinary)Chloride complexation of the group-4 elements Zr and Hf in 8.0-11.9 M HCl is investigated by Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy to characterize chloro complexes of the transactinide element, rutherfordium (Rf). The complexes of Zr and Hf successively vary with the concentration of HCl from a hydrated complex [M(H O)
O)  ]
] at 8.0 M to a hexachloro complex [MCl
at 8.0 M to a hexachloro complex [MCl ]
] at 11.9 M (M = Zr and Hf). The present structural changes of the Zr and Hf complexes well reflect the previously studied anion-exchange behavior of Zr and Hf in HCl. From both the EXAFS and anion-exchange results, we suggest that Rf forms the same complexes as those of Zr and Hf in HCl, and that the complexation strength of the hexachloro complexes of the group-4 elements, [MCl
at 11.9 M (M = Zr and Hf). The present structural changes of the Zr and Hf complexes well reflect the previously studied anion-exchange behavior of Zr and Hf in HCl. From both the EXAFS and anion-exchange results, we suggest that Rf forms the same complexes as those of Zr and Hf in HCl, and that the complexation strength of the hexachloro complexes of the group-4 elements, [MCl ]
]  (M = Zr, Hf, and Rf), is in the sequence of Rf
(M = Zr, Hf, and Rf), is in the sequence of Rf  Zr
Zr  Hf.
Hf.
Wada, Atsushi*; Watanabe, Masayuki; Yamanoi, Yoshinori*; Nankawa, Takuya; Namiki, Kosuke*; Yamasaki, Mikio*; Murata, Masaki*; Nishihara, Hiroshi*
Bulletin of the Chemical Society of Japan, 80(2), p.335 - 345, 2007/02
Times Cited Count:22 Percentile:56.69(Chemistry, Multidisciplinary)Lanthanide complexes with linear and cyclic octadentate oligopyridine-amine ligands were synthesized, and their molecular structures were determined by single-crystal X-ray crystallography. All of the complexes had a distorted capped square antiprism (CSAP) geometry, and the coordination environments of lanthanide complexes were more distortedfor the complexes with the linear ligand than those with the cyclic ligand. The Eu complexes with the linear ligand showed more intense emissions, which were attributed to the
complexes with the linear ligand showed more intense emissions, which were attributed to the 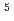 D
D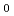
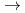
 F
F transition, than the complex withthe cyclic ligand in acetonitrile, which can be attributed to the distortion in the coordination environments. These results indicate that the coordination environments of lanthanide complexes, and thus the luminescence properties, can be controlled by tuning the geometrical structures of polydentate ligands.
transition, than the complex withthe cyclic ligand in acetonitrile, which can be attributed to the distortion in the coordination environments. These results indicate that the coordination environments of lanthanide complexes, and thus the luminescence properties, can be controlled by tuning the geometrical structures of polydentate ligands.
Hakoda, Teruyuki; Sako, Toshihiro*; Shimada, Akihiko; Ishida, Tsuneo*; Kojima, Takuji
Bulletin of the Chemical Society of Japan, 79(5), p.731 - 737, 2006/05
Times Cited Count:5 Percentile:26.30(Chemistry, Multidisciplinary)no abstracts in English