Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 CrN
CrN H
HFine, L.*; Lav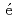 n, R.*; Wei, Z.*; Tsumori, Tatsuya*; Kageyama, Hiroshi*; Kajimoto, Ryoichi; Jimen
n, R.*; Wei, Z.*; Tsumori, Tatsuya*; Kageyama, Hiroshi*; Kajimoto, Ryoichi; Jimen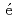 z-Ruiz, M.*; Koza, M. M.*; Karlsson, M.*
z-Ruiz, M.*; Koza, M. M.*; Karlsson, M.*
Chemistry of Materials, 37(1), p.489 - 496, 2024/12
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)Kiliyankil, V. A.*; Fukutani, Katsuyuki; 10 of others*
Journal of Materials Chemistry A, 12(42), p.28731 - 28743, 2024/10
Times Cited Count:0 Percentile:0.00(Chemistry, Physical) Ba
Ba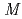 (PO
(PO )
) (
(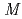 = Mg, Mn, Co, and Ni)
= Mg, Mn, Co, and Ni)Kajita, Yoichi*; Nagai, Takayuki*; Yamagishi, Shigetada*; Kimura, Kenta*; Hagihara, Masato; Kimura, Tsuyoshi*
Chemistry of Materials, 36(15), p.7451 - 7458, 2024/08
Times Cited Count:2 Percentile:56.91(Chemistry, Physical) F
FKatsumata, Tetsuhiro*; Suzuki, Ryo*; Sato, Naoto*; Oda, Ryoya*; Motoyama, Shingo*; Suzuki, Shumpei*; Nakashima, Mamoru*; Inaguma, Yoshiyuki*; Mori, Daisuke*; Aimi, Akihisa*; et al.
Chemistry of Materials, 36(8), p.3697 - 3704, 2024/04
Times Cited Count:1 Percentile:35.94(Chemistry, Physical)A perovskite-type oxynitride BaFeO F was prepared by high-pressure synthesis. Since the SHG signal was observed in the obtained material, suggesting the existence of spontaneous polarization, the mechanism of polarization was investigated by synchrotron high-energy X-ray diffraction. The obtained pair distribution functions were fitted, and a local polarization mechanism with different orientations was found. Since BaFeO
F was prepared by high-pressure synthesis. Since the SHG signal was observed in the obtained material, suggesting the existence of spontaneous polarization, the mechanism of polarization was investigated by synchrotron high-energy X-ray diffraction. The obtained pair distribution functions were fitted, and a local polarization mechanism with different orientations was found. Since BaFeO F is also a magnetic material, a magnetic domain and a ferroelectric domain are considered to coexist.
F is also a magnetic material, a magnetic domain and a ferroelectric domain are considered to coexist.
 Fe
Fe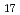 intermetallic compound
intermetallic compoundCao, Y.*; Zhou, H.*; Khmelevskyi, S.*; Lin, K.*; Avdeev, M.*; Wang, C.-W.*; Wang, B.*; Hu, F.*; Kato, Kenichi*; Hattori, Takanori; et al.
Chemistry of Materials, 35(8), p.3249 - 3255, 2023/04
Times Cited Count:2 Percentile:21.90(Chemistry, Physical)Hydrostatic and chemical pressure are efficient stimuli to alter the crystal structure and are commonly used for tuning electronic and magnetic properties in materials science. However, chemical pressure is difficult to quantify and a clear correspondence between these two types of pressure is still lacking. Here, we study intermetallic candidates for a permanent magnet with a negative thermal expansion (NTE). Based on in situ synchrotron X-ray diffraction, negative chemical pressure is revealed in Ho Fe
Fe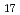 on Al doping and quantitatively evaluated by using temperature and pressure dependence of unit cell volume. A combination of magnetization and neutron diffraction measurements also allowed one to compare the effect of chemical pressure on magnetic ordering with that of hydrostatic pressure. Intriguingly, pressure can be used to control suppression and enhancement of NTE. Electronic structure calculations indicate that pressure affected the top of the majority band with respect to the Fermi level, which has implications for the magnetic stability, which in turn plays a critical role in modulating magnetism and NTE. This work presents a good example of understanding the effect of pressure and utilizing it to control properties of functional materials.
on Al doping and quantitatively evaluated by using temperature and pressure dependence of unit cell volume. A combination of magnetization and neutron diffraction measurements also allowed one to compare the effect of chemical pressure on magnetic ordering with that of hydrostatic pressure. Intriguingly, pressure can be used to control suppression and enhancement of NTE. Electronic structure calculations indicate that pressure affected the top of the majority band with respect to the Fermi level, which has implications for the magnetic stability, which in turn plays a critical role in modulating magnetism and NTE. This work presents a good example of understanding the effect of pressure and utilizing it to control properties of functional materials.
Yamagishi, Shigetada*; Hayashida, Takeshi*; Misawa, Ryusuke*; Kimura, Kenta*; Hagihara, Masato; Murata, Tomoki*; Hirose, Sakyo*; Kimura, Tsuyoshi*
Chemistry of Materials, 35(2), p.747 - 754, 2023/01
Times Cited Count:9 Percentile:67.40(Chemistry, Physical)Komatsu, Yuya*; Shimizu, Ryota*; Sato, Ryuhei*; Wilde, M.*; Nishio, Kazunori*; Katase, Takayoshi*; Matsumura, Daiju; Saito, Hiroyuki*; Miyauchi, Masahiro*; Adelman, J. R.*; et al.
Chemistry of Materials, 34(8), p.3616 - 3623, 2022/04
Times Cited Count:17 Percentile:76.57(Chemistry, Physical) GeP
GeP S
S
Yajima, Takeshi*; Hinuma, Yoyo*; Hori, Satoshi*; Iwasaki, Rui*; Kanno, Ryoji*; Ohara, Takashi; Nakao, Akiko*; Munakata, Koji*; Hiroi, Zenji*
Journal of Materials Chemistry A, 9(18), p.11278 - 11284, 2021/05
Times Cited Count:31 Percentile:81.60(Chemistry, Physical)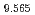 (Si
(Si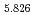 []
[]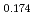 )O
)O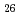 apatite without interstitial oxygens due to the overbonded channel oxygens
apatite without interstitial oxygens due to the overbonded channel oxygensFujii, Kotaro*; Yashima, Masatomo*; Hibino, Keisuke*; Shiraiwa, Masahiro*; Fukuda, Koichiro*; Nakayama, Susumu*; Ishizawa, Nobuo*; Hanashima, Takayasu*; Ohara, Takashi
Journal of Materials Chemistry A, 6(23), p.10835 - 10846, 2018/06
Times Cited Count:30 Percentile:68.19(Chemistry, Physical)Hamada, Takashi; Hasegawa, Shin; Fukasawa, Hideyuki*; Sawada, Shinichi; Koshikawa, Hiroshi; Miyashita, Atsumi; Maekawa, Yasunari
Journal of Materials Chemistry A, 3(42), p.20983 - 20991, 2015/11
Times Cited Count:36 Percentile:69.52(Chemistry, Physical)no abstracts in English
 -type giant tetragonal compound Bi
-type giant tetragonal compound Bi ZnVO
ZnVO and its stability under pressure
and its stability under pressureYu, R.*; Hojo, Hajime*; Oka, Kengo*; Watanuki, Tetsu; Machida, Akihiko; Shimizu, Keisuke*; Nakano, Kiho*; Azuma, Masaki*
Chemistry of Materials, 27(6), p.2012 - 2017, 2015/03
Times Cited Count:27 Percentile:60.78(Chemistry, Physical)no abstracts in English
 RuO
RuO epitaxial film electrodes and
epitaxial film electrodes and 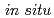 surface X-ray analysis
surface X-ray analysisTaminato, So*; Hirayama, Masaaki*; Suzuki, Kota*; Kim, K.-S.*; Zheng, Y.*; Tamura, Kazuhisa; Mizuki, Junichiro; Kanno, Ryoji*
Journal of Materials Chemistry A, 2(34), p.17875 - 17882, 2014/11
Times Cited Count:23 Percentile:55.73(Chemistry, Physical)The surface structure of a lithium-rich layered material and its relation to intercalation properties were investigated by synchrotron X-ray surface structural analyses using Li RuO
RuO epitaxial-film model electrodes with different lattice planes of (010) and (001). Electrochemical charge-discharge measurements confirmed reversible lithium intercalation activity through both planes, corresponding to three-dimensional lithium diffusion within the Li
epitaxial-film model electrodes with different lattice planes of (010) and (001). Electrochemical charge-discharge measurements confirmed reversible lithium intercalation activity through both planes, corresponding to three-dimensional lithium diffusion within the Li RuO
RuO . The (001) plane exhibited higher discharge capacities compared to the (010) plane under high rate operation (over 5 C). Direct observations of surface structural changes by
. The (001) plane exhibited higher discharge capacities compared to the (010) plane under high rate operation (over 5 C). Direct observations of surface structural changes by 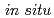 surface X-ray diffraction (XRD) and surface X-ray absorption near edge structure (XANES) established that an irreversible phase change occurs at the (010) surface during the first (de)intercalation process, whereas reversible structural changes take place at the (001) surface.
surface X-ray diffraction (XRD) and surface X-ray absorption near edge structure (XANES) established that an irreversible phase change occurs at the (010) surface during the first (de)intercalation process, whereas reversible structural changes take place at the (001) surface.
Matsumoto, Yoshihiro; Entani, Shiro; Koide, Akihiro*; Otomo, Manabu; Avramov, P.; Naramoto, Hiroshi*; Amemiya, Kenta*; Fujikawa, Takashi*; Sakai, Seiji
Journal of Materials Chemistry C, 1(35), p.5533 - 5537, 2013/09
Times Cited Count:34 Percentile:76.08(Materials Science, Multidisciplinary)Long, Y.-W.*; Kawakami, Takateru*; Chen, W.-T.*; Saito, Takashi*; Watanuki, Tetsu; Nakakura, Yuta*; Liu, Q.-Q.*; Jin, C.-Q.*; Shimakawa, Yuichi*
Chemistry of Materials, 24(11), p.2235 - 2239, 2012/06
Times Cited Count:39 Percentile:71.14(Chemistry, Physical)An A-site ordered perovskite-structure oxide, LaCu Fe
Fe O
O , shows unusual intermetallic charge transfer between the A-site Cu and the B-site Fe ions. Like temperature, pressure also induces the intermetallic charge transfer at room temperature and the compound changes from low-pressure LaCu
, shows unusual intermetallic charge transfer between the A-site Cu and the B-site Fe ions. Like temperature, pressure also induces the intermetallic charge transfer at room temperature and the compound changes from low-pressure LaCu
 Fe
Fe
 O
O to high-pressure LaCu
to high-pressure LaCu
 Fe
Fe
 O
O accompanying with significant volume collapse and as well as unusual softening in bulk modulus. In addition, the material was changed from an antiferromagnetic insulator to a paramagnetic metal transition. Either by physical or chemical (cation substitution) pressure, the charge-transfer transition temperature decreases, and the lower volume phase stabilizes Cu
accompanying with significant volume collapse and as well as unusual softening in bulk modulus. In addition, the material was changed from an antiferromagnetic insulator to a paramagnetic metal transition. Either by physical or chemical (cation substitution) pressure, the charge-transfer transition temperature decreases, and the lower volume phase stabilizes Cu and Fe
and Fe at the A and B sites, respectively.
at the A and B sites, respectively.
Fukidome, Hirokazu*; Takahashi, Ryota*; Abe, Shunsuke*; Imaizumi, Kei*; Handa, Hiroyuki*; Kang, H. C.*; Karasawa, Hiromi*; Suemitsu, Tetsuya*; Otsuji, Taiichi*; Enta, Yoshiharu*; et al.
Journal of Materials Chemistry, 21(43), p.17242 - 17248, 2011/11
Times Cited Count:29 Percentile:62.70(Chemistry, Physical) Fe
Fe O
O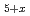 F
F (x
(x 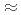 0.44)
0.44)Tsujimoto, Yoshihiro*; Yamaura, Kazunari*; Hayashi, Naoaki*; Kodama, Katsuaki; Igawa, Naoki; Matsushita, Yoshitaka*; Katsuya, Yoshio*; Shirako, Yuichi*; Akaogi, Masaki*; Muromachi, Eiji*
Chemistry of Materials, 23(16), p.3652 - 3658, 2011/08
Times Cited Count:29 Percentile:62.70(Chemistry, Physical)Topotactic reaction of the Ruddlesden-Popper phase Sr Fe
Fe O
O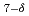 (
(
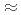 0.18) with polytetrafluoroethylene yields a highly fluorinated phase Sr
0.18) with polytetrafluoroethylene yields a highly fluorinated phase Sr Fe
Fe O
O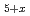 F
F (x
(x 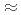 0.44), compared with Sr
0.44), compared with Sr Fe
Fe O
O F
F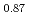 prepared by the reaction of Sr
prepared by the reaction of Sr Fe
Fe O
O and F
and F gas. Structure analyses based on powder neutron diffraction, synchrotron powder diffraction, and
gas. Structure analyses based on powder neutron diffraction, synchrotron powder diffraction, and 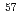 Fe M
Fe M ssbauer spectroscopy measurements demonstrate that the new oxyfluoride perovskite has no anion deficiencies and adopts the tetragonal structure (space group
ssbauer spectroscopy measurements demonstrate that the new oxyfluoride perovskite has no anion deficiencies and adopts the tetragonal structure (space group 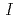 4/
4/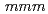 ) with lattice constants
) with lattice constants  = 3.87264(6)
= 3.87264(6)  and
and  = 21.3465(6)
= 21.3465(6)  at room temperature. The fluoride ions preferentially occupy the terminal apical anion sites with oxide ions in a disordered manner, which results in square pyramidal coordination around iron.
at room temperature. The fluoride ions preferentially occupy the terminal apical anion sites with oxide ions in a disordered manner, which results in square pyramidal coordination around iron.
 O
O glass
glassBrazhkin, V. V.*; Akola, J.*; Katayama, Yoshinori; Kohara, Shinji*; Kondrin, M. V.*; Lyapin, A. G.*; Lyapin, S. G.*; Tricot, G.*; Yagafarov, O.
Journal of Materials Chemistry, 21(28), p.10442 - 10447, 2011/07
Times Cited Count:18 Percentile:48.14(Chemistry, Physical)P O
O compound is an archetypical glass-forming oxide with a high hygroscopicity. We found that the quenching from the P
compound is an archetypical glass-forming oxide with a high hygroscopicity. We found that the quenching from the P O
O melt under ultrahigh pressures enables obtaining densified P
melt under ultrahigh pressures enables obtaining densified P O
O glasses with a residual densification up to 12% at normal conditions. These glasses have a low hygroscopicity and can exist under air conditions for several weeks. An examination of the structure of the new form of P
glasses with a residual densification up to 12% at normal conditions. These glasses have a low hygroscopicity and can exist under air conditions for several weeks. An examination of the structure of the new form of P O
O glass reveals a cardinal decrease of the volume of nanovoids in the glassy matrix.
glass reveals a cardinal decrease of the volume of nanovoids in the glassy matrix.
Enomoto, Kazuyuki*; Takahashi, Shuichi*; Iwase, Takanori*; Yamashita, Takashi*; Maekawa, Yasunari
Journal of Materials Chemistry, 21(25), p.9343 - 9349, 2011/07
Times Cited Count:39 Percentile:71.42(Chemistry, Physical)The degradation manner of graft-type polymer electrolyte membranes consisting of hydrophilic poly(styrenesulfonic acid) (PSSA) graft polymers (grafts) and thermally and mechanically stable hydrophobic polymer substrates was examined in hot water. Severe weight loss was observed in PSSA-grafted cPTFE and ETFE films but not in a PSSA-grafted PEEK film. The decomposed extracts in water were characterized as PSSA during the whole course of degradation, clearly showing that the PSSA grafts detached from the fluorinated substrates without decomposition or scission of graft polymer chains. This is quite a new degradation manner for graft-type polymer electrolyte membranes; namely, hydrophilic PSSA grafts detach from hydrophobic polymer substrates due to swelling-induced stress at the interfacial boundary in the grafted films.
Dopieralski, P.*; Anjukandi, P.*; R ckert, M.*; Shiga, Motoyuki; Ribas-Arino, J.*; Marx, D.*
ckert, M.*; Shiga, Motoyuki; Ribas-Arino, J.*; Marx, D.*
Journal of Materials Chemistry, 21(23), p.8309 - 8316, 2011/06
Times Cited Count:51 Percentile:77.88(Chemistry, Physical)The role played by polyethylene-like oligomers in transducing external tensile forces to benzocyclobutene mechanophores is investigated computationally. It is demonstrated that the oligomer chains do indeed exert a notable influence on the force dependence of the activation energies of both conrotatory and disrotatory ring-opening processes of a cis 1,2-disubstitutedbenzocyclobutene. This opens the doorway to tuning the properties of mechanoresponsive materials not only by changing the properties of the mechanophore itself, but also by tailoring the force-transducing chain molecules attached to it.
Dopieralski, P.*; Anjukandi, P.*; R ckert, M.*; Shiga, Motoyuki; Ribas-Arino, J.*; Marx, D.*
ckert, M.*; Shiga, Motoyuki; Ribas-Arino, J.*; Marx, D.*
Journal of Materials Chemistry, 21(23), p.8309 - 8316, 2011/06
The role played by polyethylene-like oligomers in transducing external tensile forces to benzo-cyclobutene mechanophores is investigated computationally. It is demonstrated that the oligomerchains do indeed exert a notable influence on the force-dependence of the activation energies of bothconrotatory and disrotatory ring-opening processes of a 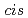 1,2-disubstituted benzocyclobutene. This opens the doorway to tuning the properties of mechanoresponsive materials not only bychanging the properties of the mechanophore itself, but also by tailoring the force-transducing chain molecules attached to it. Furthermore, it is found that these chains even have a profound impact on the topology of the force-transformed potential energy surface in the vicinity of conrotatory transition states. Hitherto unexpected and most striking is the phenomenon that some of these conrotatory transition states are found to drive the system to disrotatory products.
1,2-disubstituted benzocyclobutene. This opens the doorway to tuning the properties of mechanoresponsive materials not only bychanging the properties of the mechanophore itself, but also by tailoring the force-transducing chain molecules attached to it. Furthermore, it is found that these chains even have a profound impact on the topology of the force-transformed potential energy surface in the vicinity of conrotatory transition states. Hitherto unexpected and most striking is the phenomenon that some of these conrotatory transition states are found to drive the system to disrotatory products.