Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yano, Yoshio*; Ono, Toshikazu*; Ohara, Takashi; Hisaeda, Yoshio*
Chemistry; A European Journal, 27(71), p.17802 - 17807, 2021/12
Times Cited Count:11 Percentile:51.41(Chemistry, Multidisciplinary)Saptiama, I.*; Kaneti, Y. V.*; Yuliarto, B.*; Kumada, Hiroaki*; Tsuchiya, Kunihiko; Fujita, Yoshitaka; Malgras, V.*; Fukumitsu, Nobuyoshi*; Sakae, Takeji*; Hatano, Kentaro*; et al.
Chemistry; A European Journal, 25(18), p.4843 - 4855, 2019/03
Times Cited Count:17 Percentile:49.91(Chemistry, Multidisciplinary)The effective utilization of various biomolecules for creating a series of mesoporous boehmite ( -AlOOH) and gamma-alumina (
-AlOOH) and gamma-alumina ( -Al
-Al O
O ) nanosheets with unique hierarchical multilayered structures is demonstrated. The nature and concentration of the biomolecules strongly influence the degree of the crystallinity, the morphology, and the textural properties of the resulting
) nanosheets with unique hierarchical multilayered structures is demonstrated. The nature and concentration of the biomolecules strongly influence the degree of the crystallinity, the morphology, and the textural properties of the resulting  -AlOOH and
-AlOOH and  -Al
-Al O
O nanosheets, allowing for easy tuning. The hierarchical
nanosheets, allowing for easy tuning. The hierarchical  -AlOOH and
-AlOOH and  -Al
-Al O
O multilayered nanosheets synthesized by using biomolecules exhibit enhanced crystallinity, improved particle separation, and well-defined multilayered structures compared to those obtained without biomolecules. More impressively, these
multilayered nanosheets synthesized by using biomolecules exhibit enhanced crystallinity, improved particle separation, and well-defined multilayered structures compared to those obtained without biomolecules. More impressively, these  -AlOOH and
-AlOOH and  -Al
-Al O
O nanosheets possess high surface areas up to 425 and 371 m
nanosheets possess high surface areas up to 425 and 371 m /g, respectively, due to their mesoporous nature and hierarchical multilayered structure. When employed for molybdenum adsorption toward medical radioisotope production, the hierarchical
/g, respectively, due to their mesoporous nature and hierarchical multilayered structure. When employed for molybdenum adsorption toward medical radioisotope production, the hierarchical  -Al
-Al O
O multilayered nanosheets exhibit Mo adsorption capacities of 33.1
multilayered nanosheets exhibit Mo adsorption capacities of 33.1 40.8mg-Mo/g.
40.8mg-Mo/g.
Ozama, Eiki*; Adachi, Sadia*; Takayanagi, Toshiyuki*; Shiga, Motoyuki
Chemistry; A European Journal, 24(48), p.12716 - 12721, 2018/08
Times Cited Count:5 Percentile:17.39(Chemistry, Multidisciplinary)The structures of trivalent actinium cation in helium clusters (Ac He
He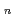 ) have been studied by quantum path integral molecular dynamics simulations with different cluster sizes,
) have been studied by quantum path integral molecular dynamics simulations with different cluster sizes,  = 18-200. The nuclear quantum effect of helium atoms plays an important role in the vibrational amplitude of the Ac
= 18-200. The nuclear quantum effect of helium atoms plays an important role in the vibrational amplitude of the Ac -He complex at low temperatures (1-3 K) where the complex is stable. We found that the coordination number of helium atoms comprising the first solvation shell can be as high as eighteen. In this case, the helium atoms are arranged in D
-He complex at low temperatures (1-3 K) where the complex is stable. We found that the coordination number of helium atoms comprising the first solvation shell can be as high as eighteen. In this case, the helium atoms are arranged in D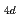 symmetry. The Ac
symmetry. The Ac -He
-He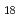 complex becomes more rigid as the cluster increases in sizes, implying that it becomes more stable. The simulation results are based on an accurate description of the Ac
complex becomes more rigid as the cluster increases in sizes, implying that it becomes more stable. The simulation results are based on an accurate description of the Ac -He interaction using relativistic ab initio calculations.
-He interaction using relativistic ab initio calculations.
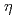
 -semiquinone complex; A Possible dielectric response mechanism
-semiquinone complex; A Possible dielectric response mechanismMitsumi, Minoru*; Ezaki, Kazunari*; Komatsu, Yuki*; Toriumi, Koshiro*; Miyato, Tatsuya*; Mizuno, Motohiro*; Azuma, Nobuaki*; Miyazaki, Yuji*; Nakano, Motohiro*; Kitagawa, Yasutaka*; et al.
Chemistry; A European Journal, 21(27), p.9682 - 9696, 2015/06
Times Cited Count:10 Percentile:31.10(Chemistry, Multidisciplinary) -isopropylacrylamide) hydrogels
-isopropylacrylamide) hydrogelsMiyamoto, Nobuyoshi*; Shimasaki, Kotaro*; Yamamoto, Kosuke*; Shintate, Morio*; Kamachi, Yuichiro*; Bastakoti, B. P.*; Suzuki, Norihiro*; Motokawa, Ryuhei; Yamauchi, Yusuke*
Chemistry; A European Journal, 20(46), p.14955 - 14958, 2014/11
Times Cited Count:18 Percentile:47.41(Chemistry, Multidisciplinary)Ribas-Arino, J.*; Shiga, Motoyuki; Marx, D.*
Chemistry; A European Journal, 15(48), p.13331 - 13335, 2009/11
Times Cited Count:44 Percentile:72.78(Chemistry, Multidisciplinary)By exploring force-transformed potential energy surfaces of both 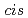 and
and 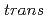 1,2-disubstitutedbenzocyclobutene molecule, we unravel the mechanism of force-induced ring-opening. The expected conrotatory process of
1,2-disubstitutedbenzocyclobutene molecule, we unravel the mechanism of force-induced ring-opening. The expected conrotatory process of 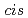 reactant leading to
reactant leading to 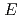 ,
, product is only observed at very small forces, whereas disrotatory ring-opening yields
product is only observed at very small forces, whereas disrotatory ring-opening yields  ,
, diene at larger forces, which is shown to be a concerted process.
diene at larger forces, which is shown to be a concerted process.