Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Rizaal, M.; Nakajima, Kunihisa
Chemosphere, 363, p.142870_1 - 142870_9, 2024/09
Tanaka, Kazuya; Yamaji, Keiko*; Masuya, Hayato*; Tomita, Jumpei; Ozawa, Mayumi*; Yamasaki, Shinya*; Tokunaga, Kohei; Fukuyama, Kenjin*; Ohara, Yoshiyuki*; Maamoun, I.*; et al.
Chemosphere, 355, p.141837_1 - 141837_11, 2024/05
In this study, biogenic Mn(IV) oxide was applied to remove Ra from mine water collected from a U mill tailings pond in the Ningyo-toge center. Just 7.6 mg of biogenic Mn(IV) oxide removed more than 98% of the 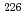 Ra from 3 L of mine water, corresponding to a distribution coefficient of 10
Ra from 3 L of mine water, corresponding to a distribution coefficient of 10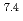 mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
Fueda, Kazuki*; Komiya, Tatsuki*; Minomo, Kenta*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Shiotsu, Hiroyuki; Onuki, Toshihiko*; Grambow, B.*; Law, G. T. W.*; et al.
Chemosphere, 328, p.138566_1 - 138566_12, 2023/07
Times Cited Count:5 Percentile:50.39(Environmental Sciences)
 removal from aqueous solutions; Cost-effectiveness & parametric effects
removal from aqueous solutions; Cost-effectiveness & parametric effectsMaamoun, I.; Eljamal, R.*; Eljamal, O.*
Chemosphere, 312, Part 1, p.137176_1 - 137176_11, 2023/01
Times Cited Count:17 Percentile:78.09(Environmental Sciences)Di Palma, A.; Adamo, P.*; Dohi, Terumi; Fujiwara, Kenso; Hagiwara, Hiroki; Kitamura, Akihiro; Sakoda, Akihiro; Sato, Kazuhiko; Iijima, Kazuki
Chemosphere, 308, Part 1, p.136179_1 - 136179_13, 2022/12
Times Cited Count:2 Percentile:11.93(Environmental Sciences)The present study shows the use of mosses transplanted in bags, called as moss bags, as biosensors of airborne radioactive dusts in the environment of the evacuated zone of Fukushima. A standardized protocol was applied and three moss species were used. Background sites of Okayama Prefecture were used for comparison. In the Fukushima area, the moss bags were able to accumulate radiocaesium in all exposure sites and periods, with Sphagnum palustre moss acting as the most performant moss. The radiocaesium activity concentrations dectected in mosses were in strong agreement with the Cs deposition levels and decontamination status of each exposure site. The accumulation of soil-derived radiocaesium by moss bags was supported by autoradiography and electron microscopy analyses. The linear dependency of Cs accumulation with the exposure time allowed a radiocaesium quantitative assessment.
Kirishima, Akira*; Terasaki, Mariko*; Miyakawa, Kazuya; Okamoto, Yoshihiro; Akiyama, Daisuke*
Chemosphere, 289, p.133181_1 - 133181_12, 2022/04
Times Cited Count:1 Percentile:3.45(Environmental Sciences)no abstracts in English
Guido-Garcia, F.; Sakamoto, Fuminori; David, K.*; Kozai, Naofumi; Grambow, B.
Chemosphere, 279, p.130511_1 - 130511_10, 2021/09
Times Cited Count:1 Percentile:3.45(Environmental Sciences)Cesium (Cs) accumulation by Shiitake was investigated to contribute to the elucidation of radiocesium-cycling mechanisms in forest environments. The results demonstrate that Shiitake non-specifically accumulates Cs while accumulating the essential element K and provide evidence that no selective Cs accumulation (or binding) sites exist within the Shiitake fruit body. Furthermore, the present results show that most accumulated Cs quickly leaches out from the dead fruit body with exposure to water. The leached Cs was largely adsorbable on clay minerals, suggesting that the Shiitake fruit body likely contains Cs in the cation form.
Kimura, Tatsuki*; Fukutani, Satoshi*; Ikegami, Maiko*; Sakamoto, Fuminori; Kozai, Naofumi; Grambow, B.*; Yoneda, Minoru*
Chemosphere, 276, p.130121_1 - 130121_7, 2021/08
Times Cited Count:2 Percentile:7.33(Environmental Sciences)The adsorption of cesium (Cs) on biotite and dissolution of Cs from Cs-bearing biotite using a siderophore were investigated aiming to contribute to the elucidation of radiocesium migration mechanisms in the soil environment. Cs was adsorbed on a hardly weathered biotite powder sample. A siderophore was extracted and purified from the bacterial culture medium, and the purified siderophore was used in five consecutive dissolution experiments of the biotite samples. The major components of the biotite (Al, Fe, and Mg) were dissolved almost stoichiometrically, strongly suggesting that the siderophore selectively dissolves the broken edges of the biotite. The Cs adsorbed on the broken edges was dissolved rapidly as the siderophore dissolved the broken edges, and then, the Cs adsorbed on the outer planar surface of the biotite particles was slowly dissolved.
Tokunaga, Kohei; Takahashi, Yoshio*; Tanaka, Kazuya; Kozai, Naofumi
Chemosphere, 266, p.129104_1 - 129104_10, 2021/03
Times Cited Count:15 Percentile:58.47(Environmental Sciences)Radioactive iodine (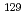 I) is of great concern owing to its high mobility in the environment and long-term radiotoxicity, but there is a lack of effective techniques for removing iodate (IO
I) is of great concern owing to its high mobility in the environment and long-term radiotoxicity, but there is a lack of effective techniques for removing iodate (IO
 ) from aqueous solution. The aim of this study is to develop a new technique for removing radioactive iodate from contaminated solution by using barite (BaSO
) from aqueous solution. The aim of this study is to develop a new technique for removing radioactive iodate from contaminated solution by using barite (BaSO ). In the present study, we examined the coprecipitation mechanism of iodate by barite at the molecular level for determining optimum conditions for iodate removal. The results showed that iodate was effectively removed from aqueous solution by coprecipitation, even in the presence of competitive anions in solution. Comparing our method with previous studies, iodate removal efficiency by barite was determined to be about two orders of magnitude greater than that by hydrotalcite-like layered double hydroxide at Cl
). In the present study, we examined the coprecipitation mechanism of iodate by barite at the molecular level for determining optimum conditions for iodate removal. The results showed that iodate was effectively removed from aqueous solution by coprecipitation, even in the presence of competitive anions in solution. Comparing our method with previous studies, iodate removal efficiency by barite was determined to be about two orders of magnitude greater than that by hydrotalcite-like layered double hydroxide at Cl concentration of 10 mmol L
concentration of 10 mmol L . Extended X-ray absorption fine structure (EXAFS) analysis indicated that incorporated iodate was strongly bound in the crystal lattice of barite by substituting the sulfate site in the structure when the iodine concentration was low. The charge compensation problem from the IO
. Extended X-ray absorption fine structure (EXAFS) analysis indicated that incorporated iodate was strongly bound in the crystal lattice of barite by substituting the sulfate site in the structure when the iodine concentration was low. The charge compensation problem from the IO
 substitution in SO
substitution in SO
 site is achieved by the substitution of Na
site is achieved by the substitution of Na -IO
-IO
 pairs at the nearest Ba
pairs at the nearest Ba site. Therefore, considering high removal efficiency and strong binding of iodate in barite, coprecipitation with barite is a promising material for removing radioactive iodate from various aqueous solutions contaminated with iodate.
site. Therefore, considering high removal efficiency and strong binding of iodate in barite, coprecipitation with barite is a promising material for removing radioactive iodate from various aqueous solutions contaminated with iodate.
 Cs for forest floor affected by the Fukushima nuclear accident; A Litterbag experiment
Cs for forest floor affected by the Fukushima nuclear accident; A Litterbag experimentSakuma, Kazuyuki; Yoshimura, Kazuya; Nakanishi, Takahiro
Chemosphere, 264, p.128480_1 - 128480_9, 2021/02
Times Cited Count:15 Percentile:58.47(Environmental Sciences)We investigated characteristic of dissolved  Cs leaching from litters collected at a coniforest needle and a deciduous broadleaf forests using litterbags at upstream area of Ohta River in Fukushima. Each leaf type of litters was collected into 36 litterbags, respectively, and installed each forest floor in June and December, 2017. Triplicate samples were collected at each forest floor and readily transported to the laboratory in August, December, 2017 and March, May, August, December, 2018. Samples were put in buckets and soaked in purified water. We took leaching water samples from the buckets at 20 min, 140 min, 1 day after soaking litter samples in the water. These samples were analysed about
Cs leaching from litters collected at a coniforest needle and a deciduous broadleaf forests using litterbags at upstream area of Ohta River in Fukushima. Each leaf type of litters was collected into 36 litterbags, respectively, and installed each forest floor in June and December, 2017. Triplicate samples were collected at each forest floor and readily transported to the laboratory in August, December, 2017 and March, May, August, December, 2018. Samples were put in buckets and soaked in purified water. We took leaching water samples from the buckets at 20 min, 140 min, 1 day after soaking litter samples in the water. These samples were analysed about  Cs activity. The main results were that the deciduous broadleaf litter showed much higher leaching ratio of dissolved
Cs activity. The main results were that the deciduous broadleaf litter showed much higher leaching ratio of dissolved  Cs (0.81-6.6%) than that of the coniferous needle litter (0.13-2.0%). A multi-regression analysis of
Cs (0.81-6.6%) than that of the coniferous needle litter (0.13-2.0%). A multi-regression analysis of  Cs leaching ratios were conducted against antecedent mean precipitation and temperature, and accumulated temperature during the litterbag experiments. The model can reproduce observed
Cs leaching ratios were conducted against antecedent mean precipitation and temperature, and accumulated temperature during the litterbag experiments. The model can reproduce observed  Cs leaching ratios (R
Cs leaching ratios (R = 0.61-0.99).
= 0.61-0.99).
 (Mosses); Towards a unified protocol of biomonitoring of airborne heavy metal pollution
(Mosses); Towards a unified protocol of biomonitoring of airborne heavy metal pollutionDi Palma, A.; Gonz lez, A. G.*; Adamo, P.*; Giordano, S.*; Reski, R.*; Pokrovsky, O. S.*
lez, A. G.*; Adamo, P.*; Giordano, S.*; Reski, R.*; Pokrovsky, O. S.*
Chemosphere, 236, p.124375_1 - 124375_9, 2019/12
Times Cited Count:13 Percentile:40.84(Environmental Sciences) Cs concentration in river water in the medium term and future following the Fukushima Nuclear accident
Cs concentration in river water in the medium term and future following the Fukushima Nuclear accidentNakanishi, Takahiro; Sakuma, Kazuyuki
Chemosphere, 215, p.272 - 279, 2019/01
Times Cited Count:55 Percentile:87.28(Environmental Sciences)We conducted a three-year-long observation (April 2015 - March 2018) of the  Cs concentration in two rivers affected by the Fukushima Dai-ichi Nuclear Power Plant (FDNPP) accident. The result revealed a declining trend for the dissolved and particulate
Cs concentration in two rivers affected by the Fukushima Dai-ichi Nuclear Power Plant (FDNPP) accident. The result revealed a declining trend for the dissolved and particulate  Cs concentration in river water in the medium term after the FDNPP accident. The dissolved and particulate
Cs concentration in river water in the medium term after the FDNPP accident. The dissolved and particulate  Cs concentrations showed declining trends with time, even though large seasonal variations related to water temperature were also observed in the dissolved
Cs concentrations showed declining trends with time, even though large seasonal variations related to water temperature were also observed in the dissolved  Cs concentrations. The environmental half-life for the dissolved
Cs concentrations. The environmental half-life for the dissolved  Cs concentration was longer than previous reported values in the early phase, suggesting that the declining trend for the dissolved
Cs concentration was longer than previous reported values in the early phase, suggesting that the declining trend for the dissolved  Cs concentration is gradually decreasing with time. The temperature dependency of the dissolved
Cs concentration is gradually decreasing with time. The temperature dependency of the dissolved  Cs concentration became weaker year by year, and the dissolved
Cs concentration became weaker year by year, and the dissolved  Cs concentration will likely remain at the same level for several decades.
Cs concentration will likely remain at the same level for several decades.
Koarashi, Jun; Nishimura, Shusaku; Atarashi-Andoh, Mariko; Matsunaga, Takeshi*; Sato, Tsutomu*; Nagao, Seiya*
Chemosphere, 205, p.147 - 155, 2018/08
Times Cited Count:21 Percentile:53.86(Environmental Sciences)There is little understanding of how soil aggregation can affect the mobility and bioavailability of  Cs in soils. To explore this, soil samples were collected at seven sites under different land-use conditions in Fukushima and were separated into four aggregate-size fractions. The fractions were then analyzed for
Cs in soils. To explore this, soil samples were collected at seven sites under different land-use conditions in Fukushima and were separated into four aggregate-size fractions. The fractions were then analyzed for  Cs content and extractability and mineral composition. In forest soils, aggregate formation was significant, and
Cs content and extractability and mineral composition. In forest soils, aggregate formation was significant, and  Cs was largely associated with large-sized aggregates. In contrast, there was less aggregation in agricultural field soils, and most of
Cs was largely associated with large-sized aggregates. In contrast, there was less aggregation in agricultural field soils, and most of  Cs was in the clay- and silt-sized fractions. Across all sites, the
Cs was in the clay- and silt-sized fractions. Across all sites, the  Cs extractability was higher in the large-sized aggregate fractions than in the clay-sized fractions. The results demonstrate that large-sized aggregates are a significant reservoir of potentially mobile and bioavailable
Cs extractability was higher in the large-sized aggregate fractions than in the clay-sized fractions. The results demonstrate that large-sized aggregates are a significant reservoir of potentially mobile and bioavailable  Cs in organic-rich (forest and orchard) soils.
Cs in organic-rich (forest and orchard) soils.
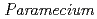 glycoprotein; Microbial transformation of heavy elements in the aquatic environment
glycoprotein; Microbial transformation of heavy elements in the aquatic environmentKozai, Naofumi; Sakamoto, Fuminori; Tanaka, Kazuya; Onuki, Toshihiko; Sato, Takahiro*; Kamiya, Tomihiro*; Grambow, B.
Chemosphere, 196, p.135 - 144, 2018/04
Times Cited Count:6 Percentile:17.62(Environmental Sciences)Transformation of heavy elements by microbes such as bacteria and fungi has been an intense research subject; however, little is known about that of protozoa. This study investigated interaction of a representative protozoa, 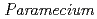 , with heavy elements (Eu(III), Pb(II), U(VI)). Non-destructive elemental analysis by micro-PIXE hardly detected those elements on living cells after sorption experiments but clearly detected on the cells that were killed with a fixative beforehand. Chromatographic analysis of aquatic species of those heavy elements after the sorption experiments revealed a fraction of those elements bound to a glycoprotein dissolved from the cell surface of living
, with heavy elements (Eu(III), Pb(II), U(VI)). Non-destructive elemental analysis by micro-PIXE hardly detected those elements on living cells after sorption experiments but clearly detected on the cells that were killed with a fixative beforehand. Chromatographic analysis of aquatic species of those heavy elements after the sorption experiments revealed a fraction of those elements bound to a glycoprotein dissolved from the cell surface of living 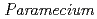 cells to form soluble pseudocolloid. These findings suggest that complexation of heavy elements with the dissolved surface glycoprotein reduced the sorption of those heavy elements on living cells.
cells to form soluble pseudocolloid. These findings suggest that complexation of heavy elements with the dissolved surface glycoprotein reduced the sorption of those heavy elements on living cells.
Kirishima, Akira*; Kuno, Atsushi*; Amamiya, Hiroki; Kubota, Takumi*; Kimuro, Shingo*; Amano, Yuki; Miyakawa, Kazuya; Iwatsuki, Teruki; Mizuno, Takashi; Sasaki, Takayuki*; et al.
Chemosphere, 168, p.798 - 806, 2017/02
Times Cited Count:3 Percentile:8.79(Environmental Sciences)For better understanding of the migration behavior of minor actinides (MA) in deep groundwater, the interaction of doped rare earth elements (REEs) and components in Horonobe deep groundwater was studied. Appx. 10 ppb of rare earth elements, i.e., Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Er, Tm and Yb were doped to the sample groundwater collected from a packed sections in borehole drilled from 140 m depth experiment drift of Horonobe underground research laboratory (URL), Hokkaido, Japan. Then, that groundwater was sequentially filtrated by 0.2 micron pore filter, 10 kDa, 3 kDa and 1 kDa of nominal molecular weight limit (NMWL) ultrafilters by keeping inert condition. After that, the filtrate solutions were analyzed by ICP-MS to determine the concentrations of retained REEs at each filtration steps, while the used filters were analyzed by the neutron activation analysis (NAA) and TOF-SIMS element mapping to know the amount and chemical speciation of trapped fraction of the REEs on each filter. A remarkable relation between the retention ratios of REEs in the filtrate solutions and the ionic radius was observed, i.e., smaller rare earth element solves more in liquid phase under the Horonobe groundwater condition. NAA and TOF-SIMS analyses revealed that certain portions of REEs were trapped by 0.2 micron pore filters as rare earth phosphates which corresponded with the predicted predominant species by a chemical equilibrium calculation for the Horonobe groundwater condition, while small portions of colloidal REEs were trapped by 10 kDa and 3 kDa NMWL ultrafilters. The result suggested that phosphate anion plays an important role in the chemical behavior of REEs in saline (seawater based) groundwater, which could be referred for the prediction of migration behavior of trivalent actinide released from the repository of radioactive waste in far future.
Koarashi, Jun; Nishimura, Shusaku; Nakanishi, Takahiro; Atarashi-Andoh, Mariko; Takeuchi, Erina; Muto, Kotomi
Chemosphere, 165, p.335 - 341, 2016/12
Times Cited Count:42 Percentile:75.32(Environmental Sciences)We established field lysimeters in a Japanese deciduous broad-leaved forest soon after the Fukushima nuclear accident to continuously monitor the downward transfer of  Cs at three depths: the litter-mineral soil boundary and depths of 5 cm and 10 cm in the mineral soil. Observations were conducted at two sites within the forest from May 2011 to May 2015. Results revealed similar temporal and depth-wise variations in
Cs at three depths: the litter-mineral soil boundary and depths of 5 cm and 10 cm in the mineral soil. Observations were conducted at two sites within the forest from May 2011 to May 2015. Results revealed similar temporal and depth-wise variations in  Cs downward fluxes for both sites. The
Cs downward fluxes for both sites. The  Cs downward fluxes generally decreased year by year at all depths, indicating that
Cs downward fluxes generally decreased year by year at all depths, indicating that  Cs was rapidly leached from the forest-floor litter layer and was then immobilized in the upper (0-5 cm) mineral soil layer through its interaction with clay minerals. The decreased inventory of mobile (or bioavailable)
Cs was rapidly leached from the forest-floor litter layer and was then immobilized in the upper (0-5 cm) mineral soil layer through its interaction with clay minerals. The decreased inventory of mobile (or bioavailable)  Cs observed during early stages after deposition indicates that the litter-soil system in the Japanese deciduous forest provides only a temporary source for
Cs observed during early stages after deposition indicates that the litter-soil system in the Japanese deciduous forest provides only a temporary source for  Cs recycling in plants.
Cs recycling in plants.
Matsunaga, Takeshi; Ueno, Takashi; R.Chandradjith*; Amano, Hikaru; ;
Chemosphere, 39(2), p.269 - 283, 1999/00
Times Cited Count:13 Percentile:34.09(Environmental Sciences)no abstracts in English