Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Uchida, Shunsuke*; Hanawa, Satoshi; Naito, Masanori*; Okada, Hidetoshi*; Lister, D. H.*
Corrosion Engineering, Science and Technology, 52(8), p.587 - 595, 2017/10
Times Cited Count:4 Percentile:18.63(Materials Science, Multidisciplinary)Based on the relationship among ECP, metal surface conditions, exposure time and other environmental conditions, a model to evaluate the ECP and corrosion rate of steel was developed by coupling a static electrochemical analysis and a dynamic oxide layer growth analysis. Major conclusion obtained on the model are as follows. The effect of H O
O and O
and O concentrations on ECP were successfully explained as the effects of oxide layer growth. Hysteresis of ECP under changes in water chemistry conditions were successfully explained with the model. Decreases in ECP due to neutron exposure were explained well by radiation-induced diffusion in the oxide layers.
concentrations on ECP were successfully explained as the effects of oxide layer growth. Hysteresis of ECP under changes in water chemistry conditions were successfully explained with the model. Decreases in ECP due to neutron exposure were explained well by radiation-induced diffusion in the oxide layers.
Sakamaki, Keiko; Kataoka, Masaharu; Maeda, Toshikatsu; Iida, Yoshihisa; Kamoshida, Michio; Yamaguchi, Tetsuji; Tanaka, Tadao
Corrosion Engineering, Science and Technology, 49(6), p.450 - 454, 2014/09
Times Cited Count:1 Percentile:0.00(Materials Science, Multidisciplinary)Corrosion experiments of a carbon steel plate embedded in bentonite mixture were conducted toverify our models assessing Eh evolution induced by corrosion of carbon steel overpack. Theexperimental results showed that the Eh decreased for the first 200 days and was subsequentlystabilised at around -450 mV; corrosion products were identified as magnetite and Fe waspresent mostly as divalent Fe within a 5 mm range from the carbon steel plate. Reactive transportmodelling was performed to assess the Eh evolution in the system using kinetic dissolution modelfor metallic iron and thermodynamic equilibrium models for other chemical reactions and closelyreproduced the experimental results. The models were verified only under the conditionsemployed in this study.
Kubo, Shinji; Futakawa, Masatoshi; Onuki, Kaoru; Yamaguchi, Akihisa*
Corrosion Engineering, 62(3), p.104 - 111, 2013/03
The iodine-sulfur thermochemical cycle for hydrogen production takes place in very harsh environments. Structural metallic materials for the hydrogen iodide decomposition are exposed in a high temperature halogen corrosion and hydrogen embrittlement environment. To evaluate adaptability of the materials, the corrosion rates and mechanical properties (the yield strength, the tensile strength, and the elongation) were measured. Prepared test specimens were exposed to ambient gas consisting of HI, I , H
, H O, and H
O, and H (molar fraction of 1:1:6:0.16) at 450
(molar fraction of 1:1:6:0.16) at 450 C for 1000 h at atmospheric pressure. After the exposure, the corrosion rates were obtained by the weight loss of each specimen. Nickel-based alloys (Hastelloy C-276, MAT21, Inconel 625) exhibited appropriate corrosion resistance (
C for 1000 h at atmospheric pressure. After the exposure, the corrosion rates were obtained by the weight loss of each specimen. Nickel-based alloys (Hastelloy C-276, MAT21, Inconel 625) exhibited appropriate corrosion resistance ( 0.03 g m
0.03 g m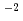 h
h . In addition, no degradations of the mechanical properties for the MAT21 and the Inconel 625 were observed. The specimens of tantalum and titanium showed hydrogen embrittlement; the specimens of zirconium and niobium exhibited poor corrosion resistance. The specimens of molybdenum (Mo) exhibited good corrosion resistance; however, the strength degradation of Mo is cause for concern. As the results show, the nickel-based alloys are well suited for the structural materials within this environment from the viewpoint of the corrosion resistance. MAT21 among them is an outstanding material with an eye to its corrosion resistance and mechanical properties.
. In addition, no degradations of the mechanical properties for the MAT21 and the Inconel 625 were observed. The specimens of tantalum and titanium showed hydrogen embrittlement; the specimens of zirconium and niobium exhibited poor corrosion resistance. The specimens of molybdenum (Mo) exhibited good corrosion resistance; however, the strength degradation of Mo is cause for concern. As the results show, the nickel-based alloys are well suited for the structural materials within this environment from the viewpoint of the corrosion resistance. MAT21 among them is an outstanding material with an eye to its corrosion resistance and mechanical properties.
Yoshida, Eiichi; Furukawa, Tomohiro
Nuclear Corrosion Science and Engineering, p.773 - 806, 2012/00
For sodium cooled fast reactor systems, the effect of sodium environment on corrosion and mechanical properties of the structural materials have to be evaluated to maintain the material integrity throughout the plant design life. In this paper, the effect of sodium on the mechanical strength, such as creep and fatigue, which is the dominant factor of corrosion was evaluated based on the related R&D results. Furthermore, the friction and self-welding phenomena in sodium were also described.
Kobayashi, Masato*; Yokoyama, Yutaka*; Takahashi, Rieko*; Asano, Hidekazu*; Taniguchi, Naoki; Naito, Morimasa
Corrosion Engineering, Science and Technology, 46(2), p.212 - 216, 2011/04
Times Cited Count:4 Percentile:27.82(Materials Science, Multidisciplinary)The corrosion behaviour of a carbon steel weld joint under anaerobic conditions was investigated to estimate the long-term integrity of the carbon steel overpack. The weld specimens in this study were produced using three welding methods: GTAW, GMAW and EBW. General corrosion was observed for each immersion specimen and the weld joint corrosion rate was the same as or less than that of the base metal. The hydrogen concentration absorbed during immersion testing was less than 2.48 10
10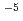 mol kg[Fe]
mol kg[Fe] (0.05 ppm) after three years, a value regarded as having little influence on hydrogen embrittlement. The susceptibility to hydrogen embrittlement was highest in the base metal, suggesting that there was little adverse effect on the weld joint from welding. The welded carbon steel overpack is assumed to maintain its resistance to corrosion as a disposal container for the expected lifetime under anaerobic underground conditions.
(0.05 ppm) after three years, a value regarded as having little influence on hydrogen embrittlement. The susceptibility to hydrogen embrittlement was highest in the base metal, suggesting that there was little adverse effect on the weld joint from welding. The welded carbon steel overpack is assumed to maintain its resistance to corrosion as a disposal container for the expected lifetime under anaerobic underground conditions.
Taniguchi, Naoki; Suzuki, Hiroyuki; Kawasaki, Manabu; Naito, Morimasa; Kobayashi, Masato*; Takahashi, Rieko*; Asano, Hidekazu*
Corrosion Engineering, Science and Technology, 46(2), p.117 - 123, 2011/04
Times Cited Count:10 Percentile:47.91(Materials Science, Multidisciplinary)Carbon steel has been selected as one of the candidate materials for overpack for geological disposal of high-level radioactive waste in Japan. Corrosion of carbon steel is divided into two types; general corrosion and localized corrosion. In this study, propagation behaviors of general and localized corrosions (pitting corrosion and crevice corrosion) were investigated by immersion tests of carbon steel under aerobic condition. The results of the immersion tests showed that the growth rate of corrosion was strongly dependent on the environmental condition and steel type, but the upper limit of pitting factor (the ratio of the maximum corrosion depth and the average corrosion depth) was approximately determined by only average corrosion depth. Based on these experimental data and literature data, an empirical model that predicts the maximum corrosion depth of an overpack from average corrosion depth was developed by applying the extreme value statistical analysis using the Gumbel distribution function.
Kawasaki, Manabu; Taniguchi, Naoki; Naito, Morimasa
Corrosion Engineering, 58(11), p.465 - 482, 2009/11
In order to clarify the influence of environmental factors on the corrosion behavior of copper overpacks in oxidizing environment, potentiodynamic and potentiostatic anodic polarization tests were performed in carbonate aqueous solutions at 80  C. As the results, the passivation was promoted and film breakdown was suppressed in higher carbonate concentrations, in lower chloride ion concentrations, and in higher pH conditions. The sulfate ion tended to promote the film breakdown of copper. The effects of the composition of the test solutions on the anodic polarization curve of copper in bentonite/sand mixture were quite smaller than those in simple aqueous solution. By comparison with previous data for lower temperature condition, it was clarified that passivation of copper was promoted in higher temperature condition, but breakdown potential, Eb was independent of temperature. The Eb, was expressed as a function of the ratio of aggressive ion and inhibiting ion such as [Cl
C. As the results, the passivation was promoted and film breakdown was suppressed in higher carbonate concentrations, in lower chloride ion concentrations, and in higher pH conditions. The sulfate ion tended to promote the film breakdown of copper. The effects of the composition of the test solutions on the anodic polarization curve of copper in bentonite/sand mixture were quite smaller than those in simple aqueous solution. By comparison with previous data for lower temperature condition, it was clarified that passivation of copper was promoted in higher temperature condition, but breakdown potential, Eb was independent of temperature. The Eb, was expressed as a function of the ratio of aggressive ion and inhibiting ion such as [Cl ]/[HCO
]/[HCO
 ] and [SO
] and [SO
 ]/[HCO
]/[HCO
 ], and it was confirmed that the Eb was lowered with increasing the ratio. When the ratio exceeds a certain value, the Eb was no longer able to be determined since the anodic poralization curve becomes active dissolution type. The lower limit of Eb in passive type region was estimated to be about -200 mV vs. SCE. The results of potentiostatic tests showed that pitting corrosion or non-uniform corrosion was observed at the potentials over Eb or second current peak potentials in anodic polarization curve.
], and it was confirmed that the Eb was lowered with increasing the ratio. When the ratio exceeds a certain value, the Eb was no longer able to be determined since the anodic poralization curve becomes active dissolution type. The lower limit of Eb in passive type region was estimated to be about -200 mV vs. SCE. The results of potentiostatic tests showed that pitting corrosion or non-uniform corrosion was observed at the potentials over Eb or second current peak potentials in anodic polarization curve.
Mori, Hiroaki*; Katsuyama, Jinya; Mochizuki, Masahito*; Nishimoto, Kazutoshi*; Toyoda, Masao*
Corrosion Engineering, 56(12), p.757 - 770, 2007/09
In order to clarify the effects of residual stress and hardening on intergranular stress corrosion cracking (IGSCC) behavior in the welds of Type 316L stainless steel with surface hardening, the residual stress and hardness in the butt joint of pipes were estimated and grain boundary sliding was analyzed from the viewpoint of microdeformation. The residual stress and hardness in hard-machined surfaces near welds was clarified from experiment and analysis method. Grain boundary sliding in the cold-rolled specimen occurs in smaller strain conditions than that in as-received specimen; the amount of grain boundary sliding increases remarkably with an increase in rolling reduction. We also clarified that grain boundary energy is raised by grain boundary sliding. On the basis of the results, we concluded that the cause of IGSCC in the welds of Type 316L stainless steel with surface hardening is the increase in grain boundary energy induced by residual stress of welding and surface hardening.
Kato, Chiaki; Yano, Masaya*; Kiuchi, Kiyoshi; Sugimoto, Katsuhisa*
Corrosion Engineering, 52(1), p.53 - 67, 2003/01
The effects of heat-transfer on the corrosion of zirconium was examined in boiling nitric acid solutions with various concentrations. Corrosion mass losses and electrochemical polarization curves were measured on the heat-transfer and isothermal surfaces in the solutions. It was found that the corrosion rate of zirconium was higher on the heat-transfer surface than that on the isothermal surface. The rate increased with increasing nitric acid concentration and solution temperature. The increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition on the surface and the removal of the decomposition product from solution by boiling bubbles. The redox potential of 12 mol/dm nitric acid on a boiling heat-transfer surface was very close to the breakdown potential of primary passivity of zirconium. This suggests the initiation of SCC on a boiling heat-transfer surface in a nuclear fuel reprocessing.
nitric acid on a boiling heat-transfer surface was very close to the breakdown potential of primary passivity of zirconium. This suggests the initiation of SCC on a boiling heat-transfer surface in a nuclear fuel reprocessing.
Kato, Chiaki; Kiuchi, Kiyoshi; Sugimoto, Katsuhisa*
Corrosion Engineering, 52(1), p.69 - 85, 2003/01
It is necessary to know the generation mechanism of high equilibrium potential in the solutions. Existing nitrogen oxides in nitric acid solutions were first analyzed by Raman spectroscopy and then existing amount of nitrogen oxides were examined by thermodynamic calculation using the SOLGASMIX software. The Raman spectroscopic analysis showed that the existing amount of un-dissociated HNO increased with increasing nitric acid concentration and solution temperature. The thermodynamic calculation showed that the important nitrogen oxides in nitric acid solutions are HNO
increased with increasing nitric acid concentration and solution temperature. The thermodynamic calculation showed that the important nitrogen oxides in nitric acid solutions are HNO , NO
, NO
 , HNO
, HNO , NO
, NO , and NO. The equilibrium potential of nitric acid solutions is, however, mainly decided by the HNO
, and NO. The equilibrium potential of nitric acid solutions is, however, mainly decided by the HNO /HNO
/HNO equilibrium. The thermodynamic calculation also suggested that the increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition of nitrous acid on the surface and the continuous removal of decomposition product from the solutions by boiling bubbles.
equilibrium. The thermodynamic calculation also suggested that the increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition of nitrous acid on the surface and the continuous removal of decomposition product from the solutions by boiling bubbles.
; Nakajima, Hajime; ; ; Kondo, Tatsuo
Corrosion Fatigue; Mechanics, Metallurgy, Electrochemistry and Engineering, p.256 - 286, 1983/00
no abstracts in English