Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 C
CPham, V. H.; Kurata, Masaki; Nagae, Yuji; Ishibashi, Ryo*; Sasaki, Masana*
Corrosion Science, 255, p.113098_1 - 113098_9, 2025/10
Times Cited Count:0Soma, Yasutaka; Komatsu, Atsushi; Kaji, Yoshiyuki; Yamamoto, Masahiro*; Igarashi, Takahiro
Corrosion Science, 251, p.112897_1 - 112897_15, 2025/07
Times Cited Count:1 Percentile:0.00(Materials Science, Multidisciplinary)Experimental and modeling studies of the oxygen ingression at the crevices of stainless steels were conducted in high-temperature water (288 C). The limiting distance of oxygen ingression,
C). The limiting distance of oxygen ingression, 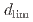 , was defined as the point beyond which the primary surface oxide changed (hematite
, was defined as the point beyond which the primary surface oxide changed (hematite  magnetite), regardless of crevice gap, oxygen concentration, and time. In situ measurements revealed increased electrical conductivity around the
magnetite), regardless of crevice gap, oxygen concentration, and time. In situ measurements revealed increased electrical conductivity around the 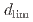 position indicating ion enrichment due to a differential oxygen concentration cell.
position indicating ion enrichment due to a differential oxygen concentration cell. 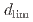 increased with increasing crevice gap, oxygen concentration, and immersion time. Modeling study suggested that oxide layer growth reduced anodic dissolution and slowed oxygen consumption, allowing oxygen ingression with time.
increased with increasing crevice gap, oxygen concentration, and immersion time. Modeling study suggested that oxide layer growth reduced anodic dissolution and slowed oxygen consumption, allowing oxygen ingression with time.
 C
CMohamad, A. B.; Nemoto, Yoshiyuki; Furumoto, Kenichiro*; Okada, Yuji*; Sato, Daiki*
Corrosion Science, 224, p.111540_1 - 111540_15, 2023/11
Times Cited Count:6 Percentile:52.51(Materials Science, Multidisciplinary) to the inside of the crevice by chelation and its effect on crevice corrosion of Type 316L stainless steel
to the inside of the crevice by chelation and its effect on crevice corrosion of Type 316L stainless steelAoyama, Takahito; Kato, Chiaki
Corrosion Science, 210(2), p.110850_1 - 110850_10, 2023/01
Times Cited Count:7 Percentile:43.24(Materials Science, Multidisciplinary)The chelated complex of Cu : [Cu(EDTA)]
: [Cu(EDTA)] was used to introduce Cu
was used to introduce Cu from outside to the inside of crevice. The introduced Cu
from outside to the inside of crevice. The introduced Cu was expected to act as an inhibitor for the crevice corrosion on stainless steels. Crevice corrosion tests, confirmed the introduction of Cu
was expected to act as an inhibitor for the crevice corrosion on stainless steels. Crevice corrosion tests, confirmed the introduction of Cu to the inside of the crevice via electromigration of [Cu(EDTA)]
to the inside of the crevice via electromigration of [Cu(EDTA)] was confirmed. Migrated [Cu(EDTA)]
was confirmed. Migrated [Cu(EDTA)] reacted with H
reacted with H and inhibited decrease in pH inside the crevice, where Cu
and inhibited decrease in pH inside the crevice, where Cu was separated from [Cu(EDTA)]
was separated from [Cu(EDTA)] and suppressed active dissolution of the stainless steel.
and suppressed active dissolution of the stainless steel.
Haoran, W.*; Yu, H.*; Liu, J.*; Kondo, Sosuke*; Okubo, Nariaki; Kasada, Ryuta*
Corrosion Science, 209, p.110818_1 - 110818_12, 2022/12
Times Cited Count:16 Percentile:78.45(Materials Science, Multidisciplinary)The corrosion behavior of newly developed Al O
O forming high Mn oxide dispersion strengthened (ODS) austenitic steels was examined in oxygen-saturated lead-bismuth eutectic at 450
forming high Mn oxide dispersion strengthened (ODS) austenitic steels was examined in oxygen-saturated lead-bismuth eutectic at 450 C for 430 h. Compared with non-ODS steels, the ODS steels possessed superior resistance to corrosion and spallation. The high density grain boundaries in the ODS steels acted as channels for the rapid outward diffusion of metallic elements, forming an internal continuous Cr
C for 430 h. Compared with non-ODS steels, the ODS steels possessed superior resistance to corrosion and spallation. The high density grain boundaries in the ODS steels acted as channels for the rapid outward diffusion of metallic elements, forming an internal continuous Cr O
O scale at the original surface. Accelerated Al diffusion, along with oxidation prevention by the external (Fe, Mn) oxide scale and the internal Cr
scale at the original surface. Accelerated Al diffusion, along with oxidation prevention by the external (Fe, Mn) oxide scale and the internal Cr O
O scale, jointly resulted in the formation of a continuous Al-rich oxide scale in ODS-7Al steel, contributing to its superior corrosion resistance.
scale, jointly resulted in the formation of a continuous Al-rich oxide scale in ODS-7Al steel, contributing to its superior corrosion resistance.
Igarashi, Takahiro; Komatsu, Atsushi; Motooka, Takafumi*; Ueno, Fumiyoshi; Yamamoto, Masahiro
Corrosion Science and Technology, 20(3), p.105 - 111, 2021/06
We constructed three dimensional computational model using cellular automata method to simulate the intergranular corrosion propagation of stainless steel. In the model, the computational system was constructed by three types of cells: grain (bulk), grain boundary (GB), and solution cell. Our simulations revealed that the surface roughness calculated by the model adopted distributed dissolution rates of GBs was greater than that adopted constant dissolution rates of GBs. The cross-sectional images obtained by our simulation were comparable with that obtained by corrosion tests. These results indicate that the surface roughness during corrosion relates the distribution of corrosion rate.
Hojo, Tomohiko*; Akiyama, Eiji*; Saito, Hiroyuki*; Shiro, Ayumi*; Yasuda, Ryo*; Shobu, Takahisa; Kinugasa, Junichiro*; Yuse, Fumio*
Corrosion Science, 177, p.108957_1 - 108957_9, 2020/12
Times Cited Count:28 Percentile:78.81(Materials Science, Multidisciplinary)Hydrogen assisted cracking on hemispherically-stretch-formed specimens of transformation induced plasticity-aided martensitic steel was investigated. Hydrogen charging induced cracking around the foot of the impression formed on the steel sheet, and the cracks propagated along the radial direction toward the hillside and the plains. Distributions of stress, plastic strain and volume fraction of retained austenite were analyzed employing the energy-dispersive X-ray diffraction method utilizing the synchrotron X-ray radiation at SPring-8. It was notable that the crack initiation took place in the region where the measured tensile stress was the highest. Influences of plastic strain and resulted martensitic transformation were also suggested.
 C
CWang, H.*; Yu, H.*; Kondo, Sosuke*; Okubo, Nariaki; Kasada, Ryuta*
Corrosion Science, 175, p.108864_1 - 108864_12, 2020/10
Times Cited Count:46 Percentile:90.71(Materials Science, Multidisciplinary)Corrosion tests were performed on newly developed alumina-forming austenitic (AFA) steels in stagnant lead bismuth eutectic (LBE) with saturated and low oxygen concentrations at 450 C for 430 h. The steels exhibited enhanced corrosion resistance to the LBE environments with the increasing of Al content. A continuous and protective Al-rich oxide scale formed on the steel specimens that were exposed to LBE with a low oxygen concentration, whereas a non-protective and stratified oxide scale formed in the oxygen saturated LBE.
C for 430 h. The steels exhibited enhanced corrosion resistance to the LBE environments with the increasing of Al content. A continuous and protective Al-rich oxide scale formed on the steel specimens that were exposed to LBE with a low oxygen concentration, whereas a non-protective and stratified oxide scale formed in the oxygen saturated LBE.
Tokita, Shun*; Kadoi, Kota*; Aoki, So; Inoue, Hiroshige*
Corrosion Science, 175, p.108867_1 - 108867_8, 2020/10
Times Cited Count:26 Percentile:77.69(Materials Science, Multidisciplinary)The purpose of this study is to evaluate the corrosion resistance of weld metal by electrochemical methods and discuss the relationship between microstructure and corrosion resistance. Intergranular and pitting corrosion resistances were measured using electrochemical potentiokinetic reactivation (EPR) test and pitting potential measurement respectively. The reactivation ratio and pitting potential corresponded to its chemical composition. The specimens containing more Cr and Mo showed higher resistance. In the EPR test, the dendrite core with a relatively low Cr content was corroded. In the pitting corrosion test, Nb carbide became the initiation site of pitting corrosion which propagated along the cell structure.
 aqueous solution
aqueous solutionIslam, M. S.*; Otani, Kyohei; Sakairi, Masatoshi*
Corrosion Science, 140, p.8 - 17, 2018/08
Times Cited Count:17 Percentile:59.10(Materials Science, Multidisciplinary)The corrosion characteristics of SUS304 exposed to 0.5M Cl aqueous solution containing different metal cations were studied with immersion tests, surface analysis and electrochemical tests. The mechanism of corrosion with metal cations was clarified by the XPS analysis results together with the hard and soft acid and base (HSAB) concept and the passive films structure. It is supposed that metal cations with large hardness make a layer by chemical bonding with the passive films. The passive films are protected by the metal cation layer from Cl
aqueous solution containing different metal cations were studied with immersion tests, surface analysis and electrochemical tests. The mechanism of corrosion with metal cations was clarified by the XPS analysis results together with the hard and soft acid and base (HSAB) concept and the passive films structure. It is supposed that metal cations with large hardness make a layer by chemical bonding with the passive films. The passive films are protected by the metal cation layer from Cl attack, and consequently corrosion reactions are inhibited.
attack, and consequently corrosion reactions are inhibited.
Uchida, Shunsuke*; Hanawa, Satoshi; Naito, Masanori*; Okada, Hidetoshi*; Lister, D. H.*
Corrosion Engineering, Science and Technology, 52(8), p.587 - 595, 2017/10
Times Cited Count:4 Percentile:18.63(Materials Science, Multidisciplinary)Based on the relationship among ECP, metal surface conditions, exposure time and other environmental conditions, a model to evaluate the ECP and corrosion rate of steel was developed by coupling a static electrochemical analysis and a dynamic oxide layer growth analysis. Major conclusion obtained on the model are as follows. The effect of H O
O and O
and O concentrations on ECP were successfully explained as the effects of oxide layer growth. Hysteresis of ECP under changes in water chemistry conditions were successfully explained with the model. Decreases in ECP due to neutron exposure were explained well by radiation-induced diffusion in the oxide layers.
concentrations on ECP were successfully explained as the effects of oxide layer growth. Hysteresis of ECP under changes in water chemistry conditions were successfully explained with the model. Decreases in ECP due to neutron exposure were explained well by radiation-induced diffusion in the oxide layers.

Rouillard, F.*; Furukawa, Tomohiro
Corrosion Science, 105, p.120 - 132, 2016/04
Times Cited Count:111 Percentile:97.23(Materials Science, Multidisciplinary)The high temperature corrosion behavior of two 9Cr and 12Cr ferritic-martensitic steel grades was studied under CO pressure varying from 1 to 250 bar for exposure times up to 8000 h. No breakaway oxidation was observed. 9Cr steel grades suffered from fast parabolic uniform oxidation and fast carburisation. Increasing CO
pressure varying from 1 to 250 bar for exposure times up to 8000 h. No breakaway oxidation was observed. 9Cr steel grades suffered from fast parabolic uniform oxidation and fast carburisation. Increasing CO pressure had very little effect on the oxidation rate but increased the carburisation rate. The corrosion behavior of both 12Cr steel grades differed and might be influenced by gas composition, minor elements or surface finish. A corrosion mechanism coupling oxidation and carburisation is proposed.
pressure had very little effect on the oxidation rate but increased the carburisation rate. The corrosion behavior of both 12Cr steel grades differed and might be influenced by gas composition, minor elements or surface finish. A corrosion mechanism coupling oxidation and carburisation is proposed.
Sakamaki, Keiko; Kataoka, Masaharu; Maeda, Toshikatsu; Iida, Yoshihisa; Kamoshida, Michio; Yamaguchi, Tetsuji; Tanaka, Tadao
Corrosion Engineering, Science and Technology, 49(6), p.450 - 454, 2014/09
Times Cited Count:1 Percentile:0.00(Materials Science, Multidisciplinary)Corrosion experiments of a carbon steel plate embedded in bentonite mixture were conducted toverify our models assessing Eh evolution induced by corrosion of carbon steel overpack. Theexperimental results showed that the Eh decreased for the first 200 days and was subsequentlystabilised at around -450 mV; corrosion products were identified as magnetite and Fe waspresent mostly as divalent Fe within a 5 mm range from the carbon steel plate. Reactive transportmodelling was performed to assess the Eh evolution in the system using kinetic dissolution modelfor metallic iron and thermodynamic equilibrium models for other chemical reactions and closelyreproduced the experimental results. The models were verified only under the conditionsemployed in this study.
Yoshida, Eiichi; Furukawa, Tomohiro
Nuclear Corrosion Science and Engineering, p.773 - 806, 2012/00
For sodium cooled fast reactor systems, the effect of sodium environment on corrosion and mechanical properties of the structural materials have to be evaluated to maintain the material integrity throughout the plant design life. In this paper, the effect of sodium on the mechanical strength, such as creep and fatigue, which is the dominant factor of corrosion was evaluated based on the related R&D results. Furthermore, the friction and self-welding phenomena in sodium were also described.
Kobayashi, Masato*; Yokoyama, Yutaka*; Takahashi, Rieko*; Asano, Hidekazu*; Taniguchi, Naoki; Naito, Morimasa
Corrosion Engineering, Science and Technology, 46(2), p.212 - 216, 2011/04
Times Cited Count:4 Percentile:27.82(Materials Science, Multidisciplinary)The corrosion behaviour of a carbon steel weld joint under anaerobic conditions was investigated to estimate the long-term integrity of the carbon steel overpack. The weld specimens in this study were produced using three welding methods: GTAW, GMAW and EBW. General corrosion was observed for each immersion specimen and the weld joint corrosion rate was the same as or less than that of the base metal. The hydrogen concentration absorbed during immersion testing was less than 2.48 10
10 mol kg[Fe]
mol kg[Fe] (0.05 ppm) after three years, a value regarded as having little influence on hydrogen embrittlement. The susceptibility to hydrogen embrittlement was highest in the base metal, suggesting that there was little adverse effect on the weld joint from welding. The welded carbon steel overpack is assumed to maintain its resistance to corrosion as a disposal container for the expected lifetime under anaerobic underground conditions.
(0.05 ppm) after three years, a value regarded as having little influence on hydrogen embrittlement. The susceptibility to hydrogen embrittlement was highest in the base metal, suggesting that there was little adverse effect on the weld joint from welding. The welded carbon steel overpack is assumed to maintain its resistance to corrosion as a disposal container for the expected lifetime under anaerobic underground conditions.
Taniguchi, Naoki; Suzuki, Hiroyuki; Kawasaki, Manabu; Naito, Morimasa; Kobayashi, Masato*; Takahashi, Rieko*; Asano, Hidekazu*
Corrosion Engineering, Science and Technology, 46(2), p.117 - 123, 2011/04
Times Cited Count:10 Percentile:47.91(Materials Science, Multidisciplinary)Carbon steel has been selected as one of the candidate materials for overpack for geological disposal of high-level radioactive waste in Japan. Corrosion of carbon steel is divided into two types; general corrosion and localized corrosion. In this study, propagation behaviors of general and localized corrosions (pitting corrosion and crevice corrosion) were investigated by immersion tests of carbon steel under aerobic condition. The results of the immersion tests showed that the growth rate of corrosion was strongly dependent on the environmental condition and steel type, but the upper limit of pitting factor (the ratio of the maximum corrosion depth and the average corrosion depth) was approximately determined by only average corrosion depth. Based on these experimental data and literature data, an empirical model that predicts the maximum corrosion depth of an overpack from average corrosion depth was developed by applying the extreme value statistical analysis using the Gumbel distribution function.
 and
and  X-ray diffractions of corrosion products freshly formed on the surface of an iron-silicon alloy
X-ray diffractions of corrosion products freshly formed on the surface of an iron-silicon alloySuzuki, Shigeru*; Matsubara, Eiichiro*; Komatsu, Takuya*; Okamoto, Yoshinori*; Kanie, Kiyoshi*; Muramatsu, Atsushi*; Konishi, Hiroyuki; Mizuki, Junichiro; Waseda, Yoshio*
Corrosion Science, 49(3), p.1081 - 1096, 2007/03
Times Cited Count:32 Percentile:79.07(Materials Science, Multidisciplinary)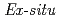 X-ray diffraction measurements of a small amount of samples extracted from wet corrosion products freshly formed on a pure iron and iron-2 mass% silicon surfaces have been conducted using synchrotron radiation. The results showed that
X-ray diffraction measurements of a small amount of samples extracted from wet corrosion products freshly formed on a pure iron and iron-2 mass% silicon surfaces have been conducted using synchrotron radiation. The results showed that  -FeOOH was formed on the outer side of wet corrosion products formed on the pure iron by sodium chloride solution, while
-FeOOH was formed on the outer side of wet corrosion products formed on the pure iron by sodium chloride solution, while  -FeOOH,
-FeOOH,  -FeOOH, Fe
-FeOOH, Fe O
O , and green rusts were formed on the inner side. In comparison to the case of the pure iron, a significant formation of
, and green rusts were formed on the inner side. In comparison to the case of the pure iron, a significant formation of  -FeOOH was observed in the iron-silicon alloy. Furthermore, in-situ diffraction measurements by a conventional X-ray source were conducted for analyzing corrosion products formed on the pure iron and iron-silicon alloy surfaces by cyclic exposure to wet and dry atmospheres. The results obtained by the
-FeOOH was observed in the iron-silicon alloy. Furthermore, in-situ diffraction measurements by a conventional X-ray source were conducted for analyzing corrosion products formed on the pure iron and iron-silicon alloy surfaces by cyclic exposure to wet and dry atmospheres. The results obtained by the  diffraction and
diffraction and 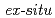 diffraction measurements on the corrosion products were consistent.
diffraction measurements on the corrosion products were consistent.
 ion implanted SUS304 stainless steel by in situ SR-XPS and ex situ scanning tunnelling microscope
ion implanted SUS304 stainless steel by in situ SR-XPS and ex situ scanning tunnelling microscopeLi, Y.; Baba, Yuji; Sekiguchi, Tetsuhiro
Corrosion Science, 43(5), p.903 - 917, 2001/05
Times Cited Count:15 Percentile:63.17(Materials Science, Multidisciplinary)no abstracts in English