Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ebihara, Mitsuru*; Shirai, Naoki*; Osawa, Takahito; Yamaguchi, Akira*
Geochimica et Cosmochimica Acta, 389, p.200 - 210, 2025/01
Times Cited Count:2 Percentile:91.17(Geochemistry & Geophysics)The 15 elements including most major elements of 12 carbonaceous chondrites collected in Antarctica were quantified using neutron-induced prompt gamma-ray analysis. While some of these samples were observed to share affinities with CI or CM chondrites, they do not exhibit the same characteristics as the known CI or CM chondrites. As a result, a new group named "CY" has been proposed for these meteorites. Common among the 12 meteorites is the depletion of volatile elements H and chlorine, showing a correlation and being depleted compared to non-Antarctic CI levels. Based on the abundance of moderately volatile elements Mn and S, the 12 meteorites can be divided into two groups: one with levels similar to non-Antarctic CI and the other with intermediate levels between CI and CM. These results suggest that CY chondrites originate from distinct parent bodies (materials), and considering two different parent bodies (materials) is necessary.
Deng, G.*; Ma, T.*; Tanaka, Kazuya; Onuki, Toshihiko*; Qiu, X.*; Yu, Q.*
Geochimica et Cosmochimica Acta, 286, p.143 - 158, 2020/10
Times Cited Count:7 Percentile:36.40(Geochemistry & Geophysics)In this study, Ce(III) adsorption on Mn(IV) oxide was investigated in the presence of dextran, one of polysaccharides. As a result, Ce(IV) on Mn(IV) oxide was solubilized by the complexation with dextran.
 and Si under anoxic and reducing conditions; Structural characteristics of ferrous silicate co-precipitates
and Si under anoxic and reducing conditions; Structural characteristics of ferrous silicate co-precipitatesFrancisco, P. C. M.; Mitsui, Seiichiro; Ishidera, Takamitsu; Tachi, Yukio; Doi, Reisuke; Shiwaku, Hideaki
Geochimica et Cosmochimica Acta, 270, p.1 - 20, 2020/02
Times Cited Count:19 Percentile:74.11(Geochemistry & Geophysics)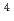 He and
He and  C dating in a granite, Tono area, central Japan
C dating in a granite, Tono area, central JapanHasegawa, Takuma*; Nakata, Kotaro*; Tomioka, Yuichi*; Goto, Kazuyuki*; Kashiwaya, Koki*; Hama, Katsuhiro; Iwatsuki, Teruki; Kunimaru, Takanori*; Takeda, Masaki
Geochimica et Cosmochimica Acta, 192, p.166 - 185, 2016/11
Times Cited Count:10 Percentile:34.95(Geochemistry & Geophysics)Groundwater dating was performed simultaneously by the 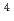 He and
He and  C methods in granite of the Tono area in central Japan. Groundwater was sampled at 30 packed-off sections of six 1000-m boreholes.
C methods in granite of the Tono area in central Japan. Groundwater was sampled at 30 packed-off sections of six 1000-m boreholes. 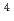 He concentrations increased and
He concentrations increased and  C concentrations decreased along a groundwater flow path on a topographic gradient.
C concentrations decreased along a groundwater flow path on a topographic gradient. 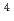 He ages were calculated by using the in situ
He ages were calculated by using the in situ 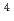 He production rate derived from the porosity, density, and U and Th content of the rock, neglecting external flux. The linear relation between the
He production rate derived from the porosity, density, and U and Th content of the rock, neglecting external flux. The linear relation between the 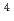 He ages and the noncorrected
He ages and the noncorrected  C ages, except in the discharge area. Simultaneous measurements make it feasible to estimate the accumulation rate of
C ages, except in the discharge area. Simultaneous measurements make it feasible to estimate the accumulation rate of 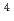 He and initial dilution of
He and initial dilution of  C, which cannot be done with a single method. Cross-checking groundwater dating has the potential to provide more reliable groundwater ages.
C, which cannot be done with a single method. Cross-checking groundwater dating has the potential to provide more reliable groundwater ages.
Togo, Yoko*; Takahashi, Yoshio*; Amano, Yuki; Matsuzaki, Hiroyuki*; Suzuki, Yohei*; Terada, Yasuko*; Muramatsu, Yasuyuki*; Ito, Kazumasa*; Iwatsuki, Teruki
Geochimica et Cosmochimica Acta, 191, p.165 - 186, 2016/10
Times Cited Count:35 Percentile:76.93(Geochemistry & Geophysics)Iodine distribution, speciation, and isotope ratio (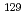 I/
I/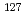 I) in both rock and groundwater phases were determined to investigate long-term migration of iodine in diatomaceous and siliceous shale. It was suggested that I
I) in both rock and groundwater phases were determined to investigate long-term migration of iodine in diatomaceous and siliceous shale. It was suggested that I is released to the ground water during the progress of the maturation of organic matter. Dissociated I
is released to the ground water during the progress of the maturation of organic matter. Dissociated I could move toward the surface because of the upward water flow driven by the compaction during burial diagenetic process. Thus, iodine rich brine is created by integration of iodine released from underlying formations. Because of low affinity of I
could move toward the surface because of the upward water flow driven by the compaction during burial diagenetic process. Thus, iodine rich brine is created by integration of iodine released from underlying formations. Because of low affinity of I to solid phase, released I
to solid phase, released I remains in solution phase, and the concentration of the iodine in the solution has been possibly increasing during sedimentation history.
remains in solution phase, and the concentration of the iodine in the solution has been possibly increasing during sedimentation history.
Yu, Q.; Onuki, Toshihiko; Tanaka, Kazuya; Kozai, Naofumi; Yamasaki, Shinya*; Sakamoto, Fuminori; Tani, Yukinori*
Geochimica et Cosmochimica Acta, 174, p.1 - 12, 2016/02
Times Cited Count:19 Percentile:56.05(Geochemistry & Geophysics)Although microorganisms possess high sorption capability for lanthanides (Lns), their biological response affecting Lns migration is unclear. We investigated the effects of microbial activity on transformation of Lns by contact of Lns with Aeremonium strictum under metabolically active condition with Mn(II). A biomolecule that specifically complex to Ce(IV) was found to be released from the fungal cell, facilitating the desorption of Ce(IV) from Mn oxide. This biomolecule was not associated with any other trivalent Lns or Fe, which differed from those non-nuclide-specific organic substances released from resting cells, as reported previously.
Onuki, Toshihiko; Jiang, M.*; Sakamoto, Fuminori; Kozai, Naofumi; Yamasaki, Shinya*; Yu, Q.; Tanaka, Kazuya; Utsunomiya, Satoshi*; Xia, X.*; Yange, K.*; et al.
Geochimica et Cosmochimica Acta, 163, p.1 - 13, 2015/08
Times Cited Count:25 Percentile:59.75(Geochemistry & Geophysics)The association of Ce(III) with the microbial cell surface and the formation of Ce phosphate nano-particles are responsible for suppressing the oxidation of Ce(III) to Ce(IV) in the mixtures.
 , Na
, Na , I
, I and HTO in compacted sodium montmorillonite as a function of porewater salinity; Integrated sorption and diffusion model
and HTO in compacted sodium montmorillonite as a function of porewater salinity; Integrated sorption and diffusion modelTachi, Yukio; Yotsuji, Kenji
Geochimica et Cosmochimica Acta, 132, p.75 - 93, 2014/05
Times Cited Count:102 Percentile:94.25(Geochemistry & Geophysics)Sorption and diffusion of radionuclides in compacted bentonite are key processes in the safe geological disposal. The effects of porewater salinity on diffusion and sorption of Cs , Na
, Na , I
, I and HTO in compacted montmorillonite were investigated by experimental and modeling approaches. Effective diffusion coefficients (De) and distribution coefficients (Kd) in montmorillonite (dry density of 800 kg/m
and HTO in compacted montmorillonite were investigated by experimental and modeling approaches. Effective diffusion coefficients (De) and distribution coefficients (Kd) in montmorillonite (dry density of 800 kg/m ) were measured under different salinities (0.01-0.5 M). The De showed cation excess and anion exclusion effects, which were influenced by salinity. The Kd values for Cs
) were measured under different salinities (0.01-0.5 M). The De showed cation excess and anion exclusion effects, which were influenced by salinity. The Kd values for Cs and Na
and Na were sensitive to salinity. The Da trends of Cs
were sensitive to salinity. The Da trends of Cs and Na
and Na are mostly independent on salinity. These results were interpreted by the integrated sorption and diffusion (ISD) model coupling the ion exchange model and the EDL diffusion model in narrow pores. This ISD model was further applied to diffusion data of monovalent cations and anions in compacted bentonite, performed well for a wide range of compaction.
are mostly independent on salinity. These results were interpreted by the integrated sorption and diffusion (ISD) model coupling the ion exchange model and the EDL diffusion model in narrow pores. This ISD model was further applied to diffusion data of monovalent cations and anions in compacted bentonite, performed well for a wide range of compaction.
Jiang, M. Y.*; Onuki, Toshihiko; Tanaka, Kazuya*; Kozai, Naofumi; Kamiishi, Eigo; Utsunomiya, Satoshi*
Geochimica et Cosmochimica Acta, 93, p.30 - 46, 2012/09
Times Cited Count:33 Percentile:64.17(Geochemistry & Geophysics)We have investigated the post-adsorption process of ytterbium (Yb) phosphate nano-particle formation by  . We have elucidated the nano particle formation by TEM and EXAFS analyses, that adsorbed Yb reacts on the cell surface with the released pohosphate from inside the cell.
. We have elucidated the nano particle formation by TEM and EXAFS analyses, that adsorbed Yb reacts on the cell surface with the released pohosphate from inside the cell.
 bound to humic substances by time-resolved laser fluorescence spectroscopy (TRLFS) and parallel factor analysis (PARAFAC)
bound to humic substances by time-resolved laser fluorescence spectroscopy (TRLFS) and parallel factor analysis (PARAFAC)Lukman, S.*; Saito, Takumi*; Aoyagi, Noboru; Kimura, Takaumi; Nagasaki, Shinya*
Geochimica et Cosmochimica Acta, 88, p.199 - 215, 2012/07
Times Cited Count:26 Percentile:55.10(Geochemistry & Geophysics) , I
, I and HTO in samples of the argillaceous Wakkanai Formation from the Horonobe URL, Japan; Clay-based modeling approach
and HTO in samples of the argillaceous Wakkanai Formation from the Horonobe URL, Japan; Clay-based modeling approachTachi, Yukio; Yotsuji, Kenji; Seida, Yoshimi*; Yui, Mikazu
Geochimica et Cosmochimica Acta, 75(22), p.6742 - 6759, 2011/11
Times Cited Count:62 Percentile:81.43(Geochemistry & Geophysics)Diffusion and sorption behaviors of Cs , I
, I and HTO in samples of the Wakkanai Formation from Horonobe URL were investigated as a function of ionic strength (IS) of synthetic groundwater by through-diffusion and batch sorption experiments. The
and HTO in samples of the Wakkanai Formation from Horonobe URL were investigated as a function of ionic strength (IS) of synthetic groundwater by through-diffusion and batch sorption experiments. The 
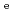 values showed cation excess and anion exclusion effects, which were strongly dependent on IS;
values showed cation excess and anion exclusion effects, which were strongly dependent on IS; 
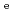 for Cs
for Cs decreased as IS increased,
decreased as IS increased, 
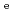 for I
for I showed the opposite dependency and
showed the opposite dependency and 
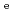 for HTO showed no dependence. The Kd values for Cs determined by through-diffusion and batch experiments were in good agreement and decreased with IS as a result of competitive ion exchange. Diffusion and sorption behaviors were interpreted based on the clay-based modeling approach in assuming the clay components of illite and smectite control diffusion and sorption mechanisms. The model predicted the
for HTO showed no dependence. The Kd values for Cs determined by through-diffusion and batch experiments were in good agreement and decreased with IS as a result of competitive ion exchange. Diffusion and sorption behaviors were interpreted based on the clay-based modeling approach in assuming the clay components of illite and smectite control diffusion and sorption mechanisms. The model predicted the 
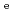 and Kd trends obtained by the series of experiments reasonably well, implying the key contribution of the clay particle and nano-size pore for ionic migration.
and Kd trends obtained by the series of experiments reasonably well, implying the key contribution of the clay particle and nano-size pore for ionic migration.
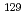 I) at the Savannah River Site?
I) at the Savannah River Site?Xu, C.*; Zhang, S.*; Ho, Y.-F.*; Miller, E. J.*; Roberts, K. A.*; Li, H.-P.*; Schwehr, K. A.*; Otosaka, Shigeyoshi; Kaplan, D. I.*; Brinkmeyer, R.*; et al.
Geochimica et Cosmochimica Acta, 75(19), p.5716 - 5735, 2011/10
Times Cited Count:65 Percentile:82.83(Geochemistry & Geophysics)In order to understand the effect of soil organic matter (SOM) on the mobility of iodine in the vicinity of the F-area seepage basin at the U.S. Department Energy's Savannah River Site (SRS), relationships between radioiodine/iodine concentration and properties of SOM (e.g., degree of humification, aromaticity, and molecular weight) were examined. Analyses were carried out for four sequential extracts of SOM (freshwater, alkaline, glycerol, and citric-alkaline solutions). Iodine in SOM was selectively bound to a small-size aromatic subunit (less than 10 kDa), and majority of water soluble 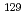 I was associated with a low molecular weight amphiphilic organic carrier (13.5-15 kDa). From these results, it was suggested that (1) SOM behaved as a sink as well as a source for iodine at the SRS, and (2) the function of SOM varies with groundwater chemistry.
I was associated with a low molecular weight amphiphilic organic carrier (13.5-15 kDa). From these results, it was suggested that (1) SOM behaved as a sink as well as a source for iodine at the SRS, and (2) the function of SOM varies with groundwater chemistry.
Takahashi, Yoshio*; Yamamoto, Mika*; Yamamoto, Yuhei; Tanaka, Kazuya
Geochimica et Cosmochimica Acta, 74(19), p.5443 - 5462, 2010/10
Times Cited Count:91 Percentile:88.37(Geochemistry & Geophysics)Among various natural samples, rare earth element (REE) pattern of bacteria exhibits anomalous enrichment at heavy REE (HREE) part, which can be a signature of bacteria-related materials. In this study, REE binding site on the cell surface of a Gram-positive bacterium (Bacillus subtilis) responsible for the HREE enrichment has been identified by extended X-ray absorption fine structure (EXAFS) coupled with the variation of REE distribution pattern. EXAFS showed that (1) HREE is bound to multiple phosphate site at lower REE-bacteria ratio (=[REE]/[bac]), but the fraction coordinated to carboxylate increased as the increase in the ratio and (2) the binding sites of light and middle REE change from phosphate with lesser coordination number to carboxylate site as the [REE]/[bac] ratio increases. On the other hand, the enrichment of HREE in the REE distribution patterns of bacteria was less marked as the increase in the [REE]/[bac] ratio. This result is consistent with the EXAFS results, since REE pattern of multiple phosphate site exhibits monotonous increase for HREE, while phosphate with lesser coordination number and carboxylate site have maxima around Sm. Based on these results, it was clear that phosphate site is more stable than carboxylate site as the binding site for REE. The average bond lengths between REE and oxygen were compared among various REE sorbed on bacteria, showing that the bond length was much shorter for HREE than those extrapolated from the trend between La and Dy due to the selective binding of HREE to the multiple phosphate site. Based on the results, it is thought that materials having such phosphate site can induce anomalous HREE enrichment in natural systems. Compared with other possible host phases of REE such as metal oxides and humic substances, the multiple phosphate site is unique to bacteria and bacteria-related materials such as biofilm and microbial mats, which leads to the potential of REE pattern as a biomarker in natural samples.
Tanaka, Kazuya; Tani, Yukinori*; Takahashi, Yoshio*; Tanimizu, Masaharu*; Suzuki, Yoshinori*; Kozai, Naofumi; Onuki, Toshihiko
Geochimica et Cosmochimica Acta, 74(19), p.5463 - 5477, 2010/10
Times Cited Count:100 Percentile:89.78(Geochemistry & Geophysics)We carried out sorption experiments of Ce by biogenic Mn oxide produced by a Mn(II)-oxidizing fungus strain KR21-2. The results of the sorption experiments indicated that Ce(III) was oxidized to Ce(IV) by biogenic Mn oxide in solution with pH 3.8 - 7. Furthermore, oxidized Ce(IV) by biogenic Mn oxide was complexed with organic ligands released from fungal cells under circumneutral conditions.

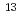 C and
C and 
 C values of
C values of 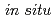 phospholipid fatty acids and carbon sources
phospholipid fatty acids and carbon sourcesMills, C.*; Amano, Yuki; Slater, G.*; Dias, R.*; Iwatsuki, Teruki; Mandernack, K.*
Geochimica et Cosmochimica Acta, 74(13), p.3785 - 3805, 2010/07
Times Cited Count:36 Percentile:64.73(Geochemistry & Geophysics)The natural abundance delta 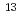 C and
C and  C values of microbial membrane phospholipid fatty acids (PLFAs) were measured and used to assess the carbon sources of bacteria in sedimentary and granitic groundwaters sampled from three boreholes in the vicinity of the Tono Uranium Mine, Gifu, Japan. The most abundant PLFA structures in all waters sampled were heterotrophic bacteria. A PLFA biomarker for type II methanotrophs in sedimentary and granitic waters, sampled from the KNA-6 borehole. The delta
C values of microbial membrane phospholipid fatty acids (PLFAs) were measured and used to assess the carbon sources of bacteria in sedimentary and granitic groundwaters sampled from three boreholes in the vicinity of the Tono Uranium Mine, Gifu, Japan. The most abundant PLFA structures in all waters sampled were heterotrophic bacteria. A PLFA biomarker for type II methanotrophs in sedimentary and granitic waters, sampled from the KNA-6 borehole. The delta  C values determined for type II methanotroph PLFAs in the groundwaters were very similar to the delta
C values determined for type II methanotroph PLFAs in the groundwaters were very similar to the delta  C values of dissolved inorganic carbon in each water. This suggests that type II methanotrophs ultimately derive all their carbon from inorganic carbon, whether directly from DIC and/or from CH
C values of dissolved inorganic carbon in each water. This suggests that type II methanotrophs ultimately derive all their carbon from inorganic carbon, whether directly from DIC and/or from CH produced by the reduction of DIC. In contrast,
produced by the reduction of DIC. In contrast, 
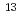 C values of type II PLFAs in the groundwaters indicate that these organisms use different carbon assimilation schemes in each environment despite very similar delta
C values of type II PLFAs in the groundwaters indicate that these organisms use different carbon assimilation schemes in each environment despite very similar delta 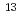 C
C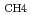 values for each water. Ancient delta
values for each water. Ancient delta  C values of some PLFAs suggest that many heterotrophs utilize ancient organic matter, perhaps from lignite seams within the sedimentary rocks.
C values of some PLFAs suggest that many heterotrophs utilize ancient organic matter, perhaps from lignite seams within the sedimentary rocks.
Wilson, J.*; Cressey, G.*; Cressey, B.*; Cuadros, J.*; Ragnarsdottir, K. V.*; Savage, D.*; Shibata, Masahiro
Geochimica et Cosmochimica Acta, 70(2), p.323 - 336, 2006/01
Times Cited Count:95 Percentile:85.70(Geochemistry & Geophysics)This work investigates montmorillonite stability in the presence of metal iron, magnetite under hydrothermal conditions. Two series of experiments were conducted. In the first, mixtures of Na-montmorillonite, native Fe, (magnetite, calcite,) and NaCl solutions were reacted at 250 C for about 100 days. In the second, mixtures of Na-montmorillonite, native Fe and FeCl
C for about 100 days. In the second, mixtures of Na-montmorillonite, native Fe and FeCl solutions were reacted at 80
solutions were reacted at 80 250
250 C for about 90 days. In the first series, the starting montmorillonite was transformed to Fe(II)-rich smectite only when the Fe metal was predominantly added. The reaction product was oxidised to Fe(III)-rich form on exposure to air. The expansion of this material on ethylene glycol solvation was much reduced compared to the starting montmorillonite. TEM imaging shows that partial loss of tetrahedral sheets, resulting in adjacent layers becoming H-bonded with a 7 angstrom repeat. Solute activities corresponded to the approximate stability field for Fe(II)-saponite. In the second series, significant smectite alteration was only observed at 250
C for about 90 days. In the first series, the starting montmorillonite was transformed to Fe(II)-rich smectite only when the Fe metal was predominantly added. The reaction product was oxidised to Fe(III)-rich form on exposure to air. The expansion of this material on ethylene glycol solvation was much reduced compared to the starting montmorillonite. TEM imaging shows that partial loss of tetrahedral sheets, resulting in adjacent layers becoming H-bonded with a 7 angstrom repeat. Solute activities corresponded to the approximate stability field for Fe(II)-saponite. In the second series, significant smectite alteration was only observed at 250 C and the product contained a small proportion of a 7
C and the product contained a small proportion of a 7 repeat structure, observable by XRD. In these experiments, solute activities coincide with berthierine.
repeat structure, observable by XRD. In these experiments, solute activities coincide with berthierine.
Wilson, J.*; Savage, D.*; Cuadros, J.*; Shibata, Masahiro; Ragnarsdottir, K. V.*
Geochimica et Cosmochimica Acta, 70(2), p.306 - 322, 2006/01
Times Cited Count:95 Percentile:58.40(Geochemistry & Geophysics)In some geological repository designs, steel canister and bentonite backfill are placed in contact. Some previous studies indicate that the montmorillonite component of the backfill could react with steel corrosion products to produce non-swelling Fe-rich phyllosilicates (e.g. chamosite, berthierine) or Fe-rich smectite. If montmorillonite were altered to non-swelling minerals, the expected swelling capacity of the bentonite backfill could be reduced. This paper discuss Fe-rich phyllosilicate mineral stability at the canister-backfill interface using thermodynamic modelling. Estimates of thermodynamic properties were made for Fe-rich clay minerals in order to construct approximate phase-relations for end-member/simplified mineral compositions in activity space. The diagrams (for the system Al O
O -FeO-Fe
-FeO-Fe O
O -MgO-Na
-MgO-Na O-SiO
O-SiO -H
-H O) suggest that if pore waters are supersaturated with magnetite, Fe(II)-rich saponite is the most likely alteration product (if fo
O) suggest that if pore waters are supersaturated with magnetite, Fe(II)-rich saponite is the most likely alteration product (if fo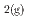 values are significantly lower than magnetite-hematite equilibrium). Therefore, the alteration of montmorillonite may not be detrimental to HLW repositories that include Fe, as long as the swelling behaviour of the Fe-rich smectite produced is maintained. If fo
values are significantly lower than magnetite-hematite equilibrium). Therefore, the alteration of montmorillonite may not be detrimental to HLW repositories that include Fe, as long as the swelling behaviour of the Fe-rich smectite produced is maintained. If fo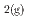 exceeds magnetite-hematite equilibrium, and solutions are saturated with magnetite, berthierine is likely to be more stable than smectite minerals. The alteration of montmorillonite to berthierine could be detrimental to the performance of the repositories.
exceeds magnetite-hematite equilibrium, and solutions are saturated with magnetite, berthierine is likely to be more stable than smectite minerals. The alteration of montmorillonite to berthierine could be detrimental to the performance of the repositories.
Onuki, Toshihiko; Ozaki, Takuo; Yoshida, Takahiro*; Sakamoto, Fuminori; Kozai, Naofumi; Wakai, Eiichi; Francis, A. J.; Iefuji, Haruyuki*
Geochimica et Cosmochimica Acta, 69(22), p.5307 - 5316, 2005/11
Times Cited Count:49 Percentile:67.29(Geochemistry & Geophysics)no abstracts in English
Takahashi, Yoshio*; Kimura, Takaumi; Minai, Yoshitaka*
Geochimica et Cosmochimica Acta, 66(1), p.1 - 12, 2002/01
Times Cited Count:63 Percentile:72.25(Geochemistry & Geophysics)no abstracts in English
 -bearing water revealed from in situ monitoring of DO, Eh and pH in a closed system
-bearing water revealed from in situ monitoring of DO, Eh and pH in a closed systemKamei, Gento; Omoto, Hiroshi*
Geochimica et Cosmochimica Acta, 64(15), p.2585 - 2601, 2000/08
Times Cited Count:86 Percentile:81.00(Geochemistry & Geophysics)None
Matsunaga, Takeshi; G.Karametaxas*; H.R.von-Gunten*; P.C.Lichtner*
Geochimica et Cosmochimica Acta, 57, p.1691 - 1704, 1993/00
Times Cited Count:75 Percentile:79.09(Geochemistry & Geophysics)no abstracts in English