Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hojo, Tomohiko*; Koyama, Motomichi*; Kumai, Bakuya*; Zhou, Y.*; Shibayama, Yuki; Shiro, Ayumi*; Shobu, Takahisa; Saito, Hiroyuki*; Ajita, Saya*; Akiyama, Eiji*
ISIJ International, 65(2), p.284 - 296, 2025/02
Times Cited Count:0 neutron diffraction measurement during tensile deformation
neutron diffraction measurement during tensile deformationYamashita, Takayuki*; Morooka, Satoshi; Gong, W.; Kawasaki, Takuro; Harjo, S.; Hojo, Tomohiko*; Okitsu, Yoshitaka*; Fujii, Hidetoshi*
ISIJ International, 64(14), p.2051 - 2060, 2024/12
Times Cited Count:0Watanabe, Miku*; Miyamoto, Goro*; Zhang, Y.*; Morooka, Satoshi; Harjo, S.; Kobayashi, Yasuhiro*; Furuhara, Tadashi*
ISIJ International, 64(9), p.1464 - 1476, 2024/07
Times Cited Count:3 Percentile:57.76(Metallurgy & Metallurgical Engineering)Kadoi, Kota*; Kanno, Yudai*; Aoki, So; Inoue, Hiroshige*
ISIJ International, 64(9), p.1450 - 1456, 2024/07
Times Cited Count:0 Percentile:0.00(Metallurgy & Metallurgical Engineering)The influence of the chemical composition on the pitting corrosion in the weld metal of austenitic stainless steel were investigated. The specimens containing higher content of chromium and molybdenum showed the lower the reactivation rate. The addition of titanium drastically deteriorated the pitting corrosion resistance. The chromium depleted region was formed near the carbide such as M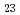 C
C and TiC. Besides, TiC phase which formed during solidification acted as nucleation sites for M
and TiC. Besides, TiC phase which formed during solidification acted as nucleation sites for M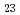 C
C precipitation. The depleted region caused by chromium diffusion because of the M
precipitation. The depleted region caused by chromium diffusion because of the M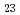 C
C precipitation, induced to deteriorate the pitting corrosion resistance.
precipitation, induced to deteriorate the pitting corrosion resistance.
Zhang, Y.*; Marusawa, Kenji*; Kudo, Kohei*; Morooka, Satoshi; Harjo, S.; Miyamoto, Goro*; Furuhara, Tadashi*
ISIJ International, 64(2), p.245 - 256, 2024/01
Times Cited Count:2 Percentile:57.76(Metallurgy & Metallurgical Engineering) neutron diffraction during cryogenic cooling
neutron diffraction during cryogenic coolingYamashita, Takayuki*; Harjo, S.; Kawasaki, Takuro; Morooka, Satoshi; Gong, W.; Fujii, Hidetoshi*; Tomota, Yo*
ISIJ International, 64(2), p.192 - 201, 2024/01
Times Cited Count:1 Percentile:0.00(Metallurgy & Metallurgical Engineering)Tsuchida, Noriyuki*; Kuramoto, Shota*; Ueji, Rintaro*; Gong, W.; Harjo, S.; Hiroi, Kosuke; Kawamura, Yukihiko*
ISIJ International, 64(2), p.354 - 360, 2024/01
Times Cited Count:0 Percentile:0.00(Metallurgy & Metallurgical Engineering)Ueji, Rintaro*; Gong, W.; Harjo, S.; Kawasaki, Takuro; Shibata, Akinobu*; Kimura, Yuji*; Inoue, Tadanobu*; Tsuchida, Noriyuki*
ISIJ International, 64(2), p.459 - 465, 2024/01
Times Cited Count:1 Percentile:36.18(Metallurgy & Metallurgical Engineering) ders deformation in an Fe-5Mn-0.1C medium-Mn steel
ders deformation in an Fe-5Mn-0.1C medium-Mn steelKoyama, Motomichi*; Yamashita, Takayuki*; Morooka, Satoshi; Sawaguchi, Takahiro*; Yang, Z.*; Hojo, Tomohiko*; Kawasaki, Takuro; Harjo, S.
ISIJ International, 62(10), p.2036 - 2042, 2022/10
Times Cited Count:23 Percentile:84.56(Metallurgy & Metallurgical Engineering) ders band propagation in an Fe-5Mn-0.1C medium Mn steel clarified through
ders band propagation in an Fe-5Mn-0.1C medium Mn steel clarified through  scanning electron microscopy
scanning electron microscopyKoyama, Motomichi*; Yamashita, Takayuki*; Morooka, Satoshi; Yang, Z.*; Varanasi, R. S.*; Hojo, Tomohiko*; Kawasaki, Takuro; Harjo, S.
ISIJ International, 62(10), p.2043 - 2053, 2022/10
Times Cited Count:12 Percentile:62.14(Metallurgy & Metallurgical Engineering)Harjo, S.; Gong, W.; Kawasaki, Takuro; Morooka, Satoshi; Yamashita, Takayuki*
ISIJ International, 62(10), p.1990 - 1999, 2022/10
Times Cited Count:2 Percentile:7.22(Metallurgy & Metallurgical Engineering)Iwamoto, Chihiro*; Takamura, Masato*; Ueno, Kota*; Kataoka, Minami*; Kurihara, Ryo*; Xu, P. G.; Otake, Yoshie*
ISIJ International, 62(5), p.1013 - 1022, 2022/05
Times Cited Count:3 Percentile:22.53(Metallurgy & Metallurgical Engineering)Amemiya, Yutaro*; Nakada, Nobuo*; Morooka, Satoshi; Kosaka, Makoto*; Kato, Masaharu*
ISIJ International, 62(2), p.282 - 290, 2022/02
Times Cited Count:3 Percentile:22.53(Metallurgy & Metallurgical Engineering)Oba, Yojiro; Morooka, Satoshi; Oishi, Kazuki*; Suzuki, Junichi*; Tsuchiyama, Toshihiro*
ISIJ International, 62(1), p.173 - 178, 2022/01
Times Cited Count:3 Percentile:22.53(Metallurgy & Metallurgical Engineering)Adachi, Nozomu*; Ueno, Haruki*; Onoe, Katsuhiko*; Morooka, Satoshi; Todaka, Yoshikazu*
ISIJ International, 61(8), p.2320 - 2322, 2021/08
Times Cited Count:5 Percentile:26.97(Metallurgy & Metallurgical Engineering)Nakayama, Yuta*; Ogawa, Fumio*; Hiyoshi, Noritake*; Hashidate, Ryuta; Wakai, Takashi; Ito, Takamoto*
ISIJ International, 61(8), p.2299 - 2304, 2021/08
Times Cited Count:5 Percentile:26.97(Metallurgy & Metallurgical Engineering)This study discusses the creep-fatigue strength for Mod.9Cr-1Mo steel at a high temperature under multiaxial loading. A low-cycle fatigue tests in various strain waveforms were performed with a hollow cylindrical specimen. The low cycle fatigue test was conducted under a proportional loading with a fixed axial strain and a non-proportional loading with a 90-degree phase difference between axial and shear strains. The low cycle fatigue tests at different strain rates and the creep-fatigue tests at different holding times were also conducted to discuss the effects of stress relaxation and strain holding on the failure life. In this study, two types of multiaxial creep-fatigue life evaluation methods were proposed: the first method is to calculate the strain range using Manson's universal slope method with considering a non-proportional loading factor and creep damage; the second method is to calculate the fatigue damage by considering the non-proportional loading factor using the linear damage law and to calculate the creep damage from the improved ductility exhaustion law. The accuracy of the evaluation methods is much better than that of the methods used in the evaluation of actual machines such as time fraction rule.
Nishimura, Hayato*; Hojo, Tomohiko*; Ajita, Saya*; Shibayama, Yuki*; Koyama, Motomichi*; Saito, Hiroyuki*; Shiro, Ayumi*; Yasuda, Ryo*; Shobu, Takahisa; Akiyama, Eiji*
ISIJ International, 61(4), p.1170 - 1178, 2021/04
Times Cited Count:8 Percentile:37.21(Metallurgy & Metallurgical Engineering)Igarashi, Takahiro; Otani, Kyohei; Kato, Chiaki; Sakairi, Masatoshi*; Togashi, Yusuke*; Baba, Kazuhiko*; Takagi, Shusaku*
ISIJ International, 61(4), p.1085 - 1090, 2021/04
Times Cited Count:2 Percentile:10.12(Metallurgy & Metallurgical Engineering)In order to clarify the effect of metal cations (Zn , Mg
, Mg , Na
, Na ) in aqueous solution on hydrogen permeation into iron, the amount of hydrogen permeation from iron surface was measured by electrochemical tests with a laser ablation. Moreover, in order to obtain the basic mechanism of hydrogen permeation with metal cation, first-principles calculations were used to acquire the adsorption potential of the metal cation and the electronic state around iron surface. By Zn
) in aqueous solution on hydrogen permeation into iron, the amount of hydrogen permeation from iron surface was measured by electrochemical tests with a laser ablation. Moreover, in order to obtain the basic mechanism of hydrogen permeation with metal cation, first-principles calculations were used to acquire the adsorption potential of the metal cation and the electronic state around iron surface. By Zn in solution, anodic reaction on ablated surface by laser irradiation was suppressed. Also, by quantum analysis Zn atoms were chemically bonded stronger than Na and Mg atoms to iron surface. It was suggested that the dissolution reaction of iron was suppressed by the formation of the Zn layer, and that lead suppression of hydrogen permeation into iron.
in solution, anodic reaction on ablated surface by laser irradiation was suppressed. Also, by quantum analysis Zn atoms were chemically bonded stronger than Na and Mg atoms to iron surface. It was suggested that the dissolution reaction of iron was suppressed by the formation of the Zn layer, and that lead suppression of hydrogen permeation into iron.
Shibayama, Yuki; Hojo, Tomohiko*; Koyama, Motomichi*; Saito, Hiroyuki*; Shiro, Ayumi*; Yasuda, Ryo*; Shobu, Takahisa; Matsuno, Takashi*; Akiyama, Eiji*
ISIJ International, 61(4), p.1322 - 1329, 2021/04
Times Cited Count:7 Percentile:37.21(Metallurgy & Metallurgical Engineering)Harjo, S.; Kawasaki, Takuro; Tsuchida, Noriyuki*; Morooka, Satoshi; Gong, W.*
ISIJ International, 61(2), p.648 - 656, 2021/02
Times Cited Count:11 Percentile:49.52(Metallurgy & Metallurgical Engineering)