Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ikeda, Yusuke*; Matsumura, Daiju; Tsuji, Takuya; Namai, Asuka*; Imoto, Kenta*; Tokoro, Hiroko*; Nakabayashi, Koji*; Okoshi, Shinichi*
Inorganica Chimica Acta, 550, p.121434_1 - 121434_8, 2023/03
Times Cited Count:0 Percentile:0.00(Chemistry, Inorganic & Nuclear)Chiera, N. M.; Sato, Tetsuya; Tomitsuka, Tomohiro; Asai, Masato; Suzuki, Hayato*; Tokoi, Katsuyuki; Toyoshima, Atsushi; Tsukada, Kazuaki; Nagame, Yuichiro
Inorganica Chimica Acta, 486, p.361 - 366, 2019/02
Times Cited Count:4 Percentile:20.86(Chemistry, Inorganic & Nuclear)The formation of NbOCl and TaOCl
and TaOCl and their adsorption behavior on quartz surfaces was explored by applying an isothermal gas-chromatographic method. Trace amounts of short-lived Nb and Ta isotopes were used. Adsorption enthalpy values (
and their adsorption behavior on quartz surfaces was explored by applying an isothermal gas-chromatographic method. Trace amounts of short-lived Nb and Ta isotopes were used. Adsorption enthalpy values ( ) at zero surface coverage of -
) at zero surface coverage of - (NbOCl
(NbOCl ) = 102
) = 102  4 kJ/mol and -
4 kJ/mol and - (TaOCl
(TaOCl ) = 128
) = 128  5 kJ/mol were determined by analyzing the chromatographic behavior of the Nb andTa complexes with a Monte-Carlo simulation method based on an adsorption-desorption kinetic model.By applying an empirical correlation, the experimental
5 kJ/mol were determined by analyzing the chromatographic behavior of the Nb andTa complexes with a Monte-Carlo simulation method based on an adsorption-desorption kinetic model.By applying an empirical correlation, the experimental  values were successively related to the macroscopic standard sublimation enthalpy,
values were successively related to the macroscopic standard sublimation enthalpy, 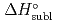 , as a measure of the volatility of each substance. The inferred sublimation enthalpies are in agreement with tabulated thermochemical values. Thus, the linear empirical correlation between
, as a measure of the volatility of each substance. The inferred sublimation enthalpies are in agreement with tabulated thermochemical values. Thus, the linear empirical correlation between  and
and 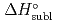 for metal-oxychlorides was updated with the inclusion of the present data. According to the predicted
for metal-oxychlorides was updated with the inclusion of the present data. According to the predicted 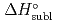 (DbOCl
(DbOCl ), a
), a  (DbOCl
(DbOCl ) value of 135
) value of 135  2 kJ/mol was extrapolated. The future accomplishment of comparative studies with DbOCl
2 kJ/mol was extrapolated. The future accomplishment of comparative studies with DbOCl under the same experimental conditions will provide valuable information on the volatility trend in Group-5 elements, together with an indication on the magnitude of relativistic effects on the electronic structure of dubnium.
under the same experimental conditions will provide valuable information on the volatility trend in Group-5 elements, together with an indication on the magnitude of relativistic effects on the electronic structure of dubnium.
 -butyl-2-pyrrolidone,
-butyl-2-pyrrolidone,  -cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone
-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidoneKoshino, Nobuyoshi*; Harada, Masayuki*; Nogami, Masanobu*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Inorganica Chimica Acta, 358(6), p.1857 - 1864, 2005/03
Times Cited Count:54 Percentile:87.98(Chemistry, Inorganic & Nuclear)Structural analyses of UO (NO
(NO )
) L
L [L=
[L= -butyl-2-pyrrolidone, N-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone] have been carried out using X-ray diffraction method. These uranyl complexes were found to have a hexagonal bipyramidal structure. The bond distances of U=O and U-O (ligand), and bond angles of U-O-C(carbonyl) are determined. In uranyl nitrate complexes with cyclic amides such as 2-pyrrolidone, urea, and caprolactam derivatives, a linear correlation was found to hold between U-O (ligand) bond distances and U-O-C(carbonyl) bond angles. Vibrational frequencies of UO
-butyl-2-pyrrolidone, N-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone] have been carried out using X-ray diffraction method. These uranyl complexes were found to have a hexagonal bipyramidal structure. The bond distances of U=O and U-O (ligand), and bond angles of U-O-C(carbonyl) are determined. In uranyl nitrate complexes with cyclic amides such as 2-pyrrolidone, urea, and caprolactam derivatives, a linear correlation was found to hold between U-O (ligand) bond distances and U-O-C(carbonyl) bond angles. Vibrational frequencies of UO (NO
(NO )
) L
L have also been measured by IR and Raman spectrophotometers. Using relationships between vibrational frequencies of O=U=O bonds and donor numbers (DNs) of ligands, donicities of N-substituted-2-pyrrolidones were determined.
have also been measured by IR and Raman spectrophotometers. Using relationships between vibrational frequencies of O=U=O bonds and donor numbers (DNs) of ligands, donicities of N-substituted-2-pyrrolidones were determined.
G.Meinrath*; Kimura, Takaumi
Inorganica Chimica Acta, 204, p.79 - 85, 1993/00
Times Cited Count:38 Percentile:84.72(Chemistry, Inorganic & Nuclear)no abstracts in English
 -CeO
-CeO solid solutions
solid solutionsHinatsu, Yukio; Fujino, Takeo
Inorganica Chimica Acta, 140, p.169 - 172, 1987/00
Times Cited Count:2 Percentile:13.63(Chemistry, Inorganic & Nuclear)no abstracts in English
Inorganica Chimica Acta, 140, p.279 - 282, 1987/00
no abstracts in English
 UF
UF and Rb
and Rb UF
UF
Iwasaki, Matae; Ishikawa, Niro; Owada, Ken; Fujino, Takeo
Inorganica Chimica Acta, 54, p.L193 - L194, 1981/00
Times Cited Count:3 Percentile:15.96(Chemistry, Inorganic & Nuclear)no abstracts in English