Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -phase in Al-Mg-Si alloys during aging treatment
-phase in Al-Mg-Si alloys during aging treatmentAhmed, A.*; Uttarasak, K.*; Tsuchiya, Taiki*; Lee, S.*; Nishimura, Katsuhiko*; Nunomura, Norio*; Shimizu, Kazuyuki*; Hirayama, Kyosuke*; Toda, Hiroyuki*; Yamaguchi, Masatake; et al.
Journal of Alloys and Compounds, 988, p.174234_1 - 174234_9, 2024/06
This study aims to clarify the growth process of the -phase in Al-Mg-Si alloys from the point of view of morphology evolution. For this research, the
-phase in Al-Mg-Si alloys from the point of view of morphology evolution. For this research, the  -phase orientation relationship, shape, growth process, misfit value, and interfacial condition between the
-phase orientation relationship, shape, growth process, misfit value, and interfacial condition between the  -phase and Al matrix were investigated using high-resolution transmission electron microscopy (HR-TEM), focus ion beam (FIB), and optical microscope (OM). Results include the identification of {111}
-phase and Al matrix were investigated using high-resolution transmission electron microscopy (HR-TEM), focus ion beam (FIB), and optical microscope (OM). Results include the identification of {111}  facets at the edges of the
facets at the edges of the  -phase, as well as the proposal of two new three-dimensional shapes for the
-phase, as well as the proposal of two new three-dimensional shapes for the  -phase. We purposed the morphology evolution during the growth process of Mg
-phase. We purposed the morphology evolution during the growth process of Mg Si crystal and calculated the misfit to understand the unstable (111)
Si crystal and calculated the misfit to understand the unstable (111) facet has a higher misfit value as compared to the (001)
facet has a higher misfit value as compared to the (001) and (011)
and (011) facets. Our observations provide how they influence the behavior of Mg
facets. Our observations provide how they influence the behavior of Mg Si crystals.
Si crystals.
 -FeNi films with island structures on LaAlO
-FeNi films with island structures on LaAlO (110) substrates by nitrogen insertion and topotactic extraction
(110) substrates by nitrogen insertion and topotactic extractionNishio, Takahiro*; Ito, Keita*; Kura, Hiroaki*; Takanashi, Koki; Yanagihara, Hideto*
Journal of Alloys and Compounds, 976, p.172992_1 - 172992_8, 2024/03
 neutron diffraction
neutron diffractionZhou, Y.*; Song, W.*; Zhang, F.*; Wu, Y.*; Lei, Z.*; Jiao, M.*; Zhang, X.*; Dong, J.*; Zhang, Y.*; Yang, M.*; et al.
Journal of Alloys and Compounds, 971, p.172635_1 - 172635_7, 2024/01
Times Cited Count:0 Percentile:0.00(Chemistry, Physical) AuGe (
AuGe ( = Y, Gd-Tm, and Lu)
= Y, Gd-Tm, and Lu)Kurumaji, Takashi*; Gen, Masaki*; Kito, Shunsuke*; Ikeuchi, Kazuhiko*; Nakamura, Mitsutaka; Ikeda, Akihiko*; Arima, Takahisa*
Journal of Alloys and Compounds, 947, p.169475_1 - 169475_8, 2023/06
Times Cited Count:1 Percentile:44.33(Chemistry, Physical) Rh
Rh Sn
Sn
Opletal, P.; Duverger-N dellec, E.*; Miliyanchuk, K.*; Malick, S.*; Hossain, Z.*; Custers, J.*
dellec, E.*; Miliyanchuk, K.*; Malick, S.*; Hossain, Z.*; Custers, J.*
Journal of Alloys and Compounds, 927, p.166941_1 - 166941_7, 2022/12
Times Cited Count:3 Percentile:40.92(Chemistry, Physical) CoCrFeNi eutectic high entropy alloy
CoCrFeNi eutectic high entropy alloyYun, D.*; Chae, H.*; Lee, T.*; Lee, D.-H.*; Ryu, H. J.*; Banerjee, R.*; Harjo, S.; Kawasaki, Takuro; Lee, S. Y.*
Journal of Alloys and Compounds, 918, p.165673_1 - 165673_7, 2022/10
Times Cited Count:3 Percentile:40.92(Chemistry, Physical)Zhang, X. X.*; Lutz, A.*; Andr , H.*; Lahres, M.*; Gong, W.; Harjo, S.; Emmelmann, C.*
, H.*; Lahres, M.*; Gong, W.; Harjo, S.; Emmelmann, C.*
Journal of Alloys and Compounds, 898, p.162890_1 - 162890_8, 2022/03
Times Cited Count:6 Percentile:66.76(Chemistry, Physical) Sc
Sc )
) O
O (0
(0  x
x  0.2) hexagonal ferrite
0.2) hexagonal ferriteMaruyama, Kenichi*; Tanaka, Seiya*; Kiyanagi, Ryoji; Nakao, Akiko*; Moriyama, Kentaro*; Ishikawa, Yoshihisa*; Amako, Yasushi*; Iiyama, Taku*; Futamura, Ryusuke*; Utsumi, Shigenori*; et al.
Journal of Alloys and Compounds, 892, p.162125_1 - 162125_8, 2022/02
Times Cited Count:2 Percentile:15.51(Chemistry, Physical)Liss, K.-D.*; Harjo, S.; Kawasaki, Takuro; Aizawa, Kazuya; Xu, P. G.
Journal of Alloys and Compounds, 869, p.159232_1 - 159232_9, 2021/07
Times Cited Count:6 Percentile:45.20(Chemistry, Physical)
Yoshii, Kenji; Ikeda, Naoshi*
Journal of Alloys and Compounds, 804, p.364 - 369, 2019/10
Times Cited Count:11 Percentile:52.97(Chemistry, Physical)Dielectric and magnetocaloric measurements are carried out for the chromite TmCrO . This oxide was reported to be multiferroic below the N
. This oxide was reported to be multiferroic below the N el temperature (
el temperature (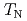 ) of 125 K, likely due to a structural transformation. The dielectric response shows large dielectric constants below 300 K. However, from the analyses of loss tangent, AC conductivity and dielectric modulus, this behavior is rooted in hopping of charge carriers rather than electric dipoles, as proposed for some other chromites. No dielectric anomaly is found at
) of 125 K, likely due to a structural transformation. The dielectric response shows large dielectric constants below 300 K. However, from the analyses of loss tangent, AC conductivity and dielectric modulus, this behavior is rooted in hopping of charge carriers rather than electric dipoles, as proposed for some other chromites. No dielectric anomaly is found at 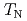 . The magnetocaloric effect shows that the magnetic transitions at
. The magnetocaloric effect shows that the magnetic transitions at 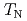 as well as the spin reorientation temperature are of a second order. This result strongly suggests the absence of magnetostructural transition at
as well as the spin reorientation temperature are of a second order. This result strongly suggests the absence of magnetostructural transition at 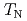 in accord with no observation of ferroelectric transition at this temperature.
in accord with no observation of ferroelectric transition at this temperature.
Iha, Wataru*; Kakihana, Masashi*; Matsuda, Shinya*; Honda, Fuminori*; Haga, Yoshinori; Takeuchi, Tetsuya*; Nakashima, Miho*; Amako, Yasushi*; Gochi, Jun*; Uwatoko, Yoshiya*; et al.
Journal of Alloys and Compounds, 788, p.361 - 366, 2019/06
Times Cited Count:6 Percentile:33.10(Chemistry, Physical)Nishimura, Katsuhiko*; Matsuda, Kenji*; Lee, S.*; Nunomura, Norio*; Shimano, Tomoki*; Bendo, A.*; Watanabe, Katsumi*; Tsuchiya, Taiki*; Namiki, Takahiro*; Toda, Hiroyuki*; et al.
Journal of Alloys and Compounds, 774, p.405 - 409, 2019/02
Times Cited Count:3 Percentile:17.35(Chemistry, Physical)Moro, Takuya*; Kim, J.*; Yamanaka, Satoru*; Murayama, Ichiro*; Kato, Takanori*; Nakayama, Tadachika*; Takeda, Masatoshi*; Yamada, Noboru*; Nishihata, Yasuo; Fukuda, Tatsuo; et al.
Journal of Alloys and Compounds, 768, p.22 - 27, 2018/11
Times Cited Count:17 Percentile:65.14(Chemistry, Physical)Hosokawa, Shinya*; Kimura, Koji*; Yamasaki, Michiaki*; Kawamura, Yoshihito*; Yoshida, Koji*; Inui, Masanori*; Tsutsui, Satoshi*; Baron, A. Q. R.*; Kawakita, Yukinobu; Ito, Shinichi*
Journal of Alloys and Compounds, 695, p.426 - 432, 2017/02
Times Cited Count:3 Percentile:16.35(Chemistry, Physical) 1
1 -type AlNi
-type AlNi alloy at high pressure and temperature
alloy at high pressure and temperatureEndo, Naruki*; Saito, Hiroyuki; Machida, Akihiko; Katayama, Yoshinori
Journal of Alloys and Compounds, 645(Suppl.1), p.S61 - S63, 2015/10
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)Kim, Jae-Hwan; Nakamichi, Masaru
Journal of Alloys and Compounds, 640, p.285 - 289, 2015/08
Times Cited Count:3 Percentile:17.70(Chemistry, Physical)Kim, Jae-Hwan; Nakamichi, Masaru
Journal of Alloys and Compounds, 638, p.277 - 281, 2015/07
Times Cited Count:5 Percentile:27.23(Chemistry, Physical)Kim, Jae-Hwan; Nakamichi, Masaru
Journal of Alloys and Compounds, 585, p.63 - 68, 2014/02
Times Cited Count:14 Percentile:55.09(Chemistry, Physical)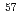 Fe M
Fe M ssbauer spectroscopy of RFe
ssbauer spectroscopy of RFe (R = Y, Gd) hydrides synthesized under ultra-high-pressure hydrogen
(R = Y, Gd) hydrides synthesized under ultra-high-pressure hydrogenMitsui, Takaya; Masuda, Ryo*; Seto, Makoto; Hirao, Naohisa*; Matsuoka, Takehiro*; Nakamura, Yumiko*; Sakaki, Koji*; Enoki, Hirotoshi*
Journal of Alloys and Compounds, 580(Suppl.1), p.S264 - S267, 2013/12
Times Cited Count:8 Percentile:42.44(Chemistry, Physical)Abe, Hiroshi; Aone, Shigeo*; Morimoto, Ryo*; Uchida, Hirohisa*
Journal of Alloys and Compounds, 580(Suppl.1), p.S219 - S221, 2013/12
Times Cited Count:5 Percentile:31.55(Chemistry, Physical)no abstracts in English