Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -isosaccharinic acid
-isosaccharinic acidKobayashi, Taishi*; Sasaki, Takayuki*; Kitamura, Akira
Journal of Chemical Thermodynamics, 138, p.151 - 158, 2019/11
Times Cited Count:6 Percentile:23.66(Thermodynamics)The effect of  -isosaccharinic acid (ISA) on the solubility and redox of tetravalent and hexavalent uranium (U(IV), U(VI)) was investigated in the hydrogen ion concentration (pH
-isosaccharinic acid (ISA) on the solubility and redox of tetravalent and hexavalent uranium (U(IV), U(VI)) was investigated in the hydrogen ion concentration (pH ) range of 6
) range of 6 13 and at total ISA concentration ([ISA]
13 and at total ISA concentration ([ISA]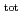 ) = 10
) = 10
 10
10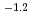 mol/dm
mol/dm . The dependence of U(IV) solubility on pH
. The dependence of U(IV) solubility on pH and [ISA]
and [ISA]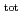 suggested the existence of U(OH)
suggested the existence of U(OH) (ISA)
(ISA)
 as a dominant species within the investigated pH
as a dominant species within the investigated pH range of 6
range of 6 12. For the U(VI)-ISA system, UO
12. For the U(VI)-ISA system, UO (OH)
(OH) (ISA)
(ISA)
 was suggested as a dominant species at pH
was suggested as a dominant species at pH 7
7 13. The formation constants of the U(IV)-ISA and U(VI)-ISA complexes were determined by least-squares fitting of the solubility data. The solubility of U(IV) and U(VI) in the presence of ISA and its effect on the redox behavior were thermodynamically interpreted based on the obtained constants.
13. The formation constants of the U(IV)-ISA and U(VI)-ISA complexes were determined by least-squares fitting of the solubility data. The solubility of U(IV) and U(VI) in the presence of ISA and its effect on the redox behavior were thermodynamically interpreted based on the obtained constants.
Kimuro, Shingo; Kirishima, Akira*; Kitatsuji, Yoshihiro; Miyakawa, Kazuya; Akiyama, Daisuke*; Sato, Nobuaki*
Journal of Chemical Thermodynamics, 132, p.352 - 362, 2019/05
Times Cited Count:16 Percentile:50.83(Thermodynamics)A combination of potentiometry and calorimetry was used for the determination of the thermodynamic quantities of complexation of generic and groundwater humic acid (HA), which was isolated from deep groundwater at Horonobe, Hokkaido, Japan, with copper (II) ions and uranyl (VI) ions. The apparent complexation constant of Horonobe HA was independent of the pH, whereas that of generic HA was dependent on the pH. This observation indicates that the polyelectrolyte effect of Horonobe HA is negligible because of its small molecular size. In addition, the effect of the heterogeneity of Horonobe HA was not significant. Moreover, the complexation enthalpy of Horonobe HA was consistent with that of homogeneous poly(acrylic acid), which means the complexation of Horonobe HA was not affected by the functional group heterogeneity. Consequently, the characteristic complexation mechanism of Horonobe HA was revealed based on the determined thermodynamic quantities.
Rai, D.*; Kitamura, Akira
Journal of Chemical Thermodynamics, 114, p.135 - 143, 2017/11
Times Cited Count:12 Percentile:20.39(Thermodynamics)Isosaccharinic acid is a cellulose degradation product that can form in low-level nuclear waste repositories and is known to form strong complexes with many elements, including actinides, disposed of in these repositories. We (1) reviewed the available data for deprotonation and lactonisation constants of isosaccharinic acid, and the isosaccharinate binding constants for Ca, Fe(III), Th, U(IV), U(VI), Np(IV), Pu(IV), and Am(III), (2) summarized complexation constant values for predicting actinide behavior in geologic repositories in the presence of isosaccharinate, and (3) outlined additional studies to acquire reliable thermodynamic data where the available data are inadequate.
Kitatsuji, Yoshihiro; Okugaki, Tomohiko*; Kasuno, Megumi*; Kubota, Hiroki*; Maeda, Koji*; Kimura, Takaumi; Yoshida, Zenko; Kihara, Sorin*
Journal of Chemical Thermodynamics, 43(6), p.844 - 851, 2011/06
Times Cited Count:8 Percentile:28.84(Thermodynamics)Standard Gibbs energies for transfer ( G
G
 ) of actinyl ions (AnO
) of actinyl ions (AnO
 ; z = 2 or 1; An: U, Np or Pu) between an aqueous solution and an organic solution were determined based on distribution method combined with voltammetry for ion transfer at the interface of two immiscible electrolyte solutions. The organic solutions examined were nitrobenzene, 1,2-dichloroethane, benzonitrile, acetophenone and 2-nitrophenyl octyl ether. Irrespective of the type of organic solutions,
; z = 2 or 1; An: U, Np or Pu) between an aqueous solution and an organic solution were determined based on distribution method combined with voltammetry for ion transfer at the interface of two immiscible electrolyte solutions. The organic solutions examined were nitrobenzene, 1,2-dichloroethane, benzonitrile, acetophenone and 2-nitrophenyl octyl ether. Irrespective of the type of organic solutions,  G
G
 of UO
of UO
 , NpO
, NpO
 and PuO
and PuO
 were nearly equal to each other and slightly larger than that of Mg
were nearly equal to each other and slightly larger than that of Mg . The
. The  G
G
 of NpO
of NpO
 was extraordinary large compared with those of ordinary monovalent cations. The dependence of
was extraordinary large compared with those of ordinary monovalent cations. The dependence of  G
G
 of AnO
of AnO
 on the type of organic solutions was similar to that of H
on the type of organic solutions was similar to that of H or Mg
or Mg . The
. The  G
G
 of An
of An and An
and An were also discussed briefly.
were also discussed briefly.
Akabori, Mitsuo; Kobayashi, Fumiaki; Hayashi, Hirokazu; Ogawa, Toru; Huntelaar, M. E.*; Booij, A. S.*; Vlaanderen, P. van*
Journal of Chemical Thermodynamics, 34(9), p.1461 - 1466, 2002/09
Times Cited Count:0 Percentile:0.00(Thermodynamics)no abstracts in English