Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Mori, Masanobu*; Sagara, Katsuya*; Arai, Kaori*; Nakatani, Nobutake*; Ohira, Shinichi*; Toda, Kei*; Itabashi, Hideyuki*; Kozaki, Daisuke*; Sugo, Yumi; Watanabe, Shigeki; et al.
Journal of Chromatography A, 1431, p.131 - 137, 2016/01
Times Cited Count:10 Percentile:38.40(Biochemical Research Methods)Shimada, Asako; Ozawa, Mayumi; Yabuki, Koshi*; Kimiyama, Kazuhiro; Sato, Kenji; Kameo, Yutaka
Journal of Chromatography A, 1371, p.163 - 167, 2014/12
Times Cited Count:16 Percentile:51.03(Biochemical Research Methods)Saito, Shingo*; Sato, Yoshiyuki*; Haraga, Tomoko; Nakano, Yuta*; Asai, Shiho; Kameo, Yutaka; Takahashi, Kuniaki; Shibukawa, Masami*
Journal of Chromatography A, 1232, p.152 - 157, 2012/04
Times Cited Count:15 Percentile:45.51(Biochemical Research Methods)A rapid and high-sensitive detection method of total concentration of Nd ion in a spent nuclear fuel sample is desirable since precise quantification of total Nd is useful as indicator of burnup. In this work, a capillary electrophoresis-laser-induced fluorescent detection method (CE-LIF) was proposed for analysis of total Nd in the spent fuel sample solution, employing a newly synthesized metal fluorescent probe with a fluorescein and a macrocylic hexadentate chelating group, FTC-ABNOTA, for lanthanide (Ln) ions. The mutual separation among the Ln-FTC-ABNOTA complexes was achieved by pH control providing dynamic ternary complexation with hydroxide ions. In this method, high resolution of Nd from other Ln ions with high resolution of 1.3-1.9 and a very low detection limit of 3.2 ppt were successfully obtained. A simulated spent fuel sample containing various metal ions was examined, so that a good quantification result with 99.3% recovery was obtained even with large excess of U.
Fujiwara, Asako; Hoshi, Akiko; Kameo, Yutaka; Nakashima, Mikio
Journal of Chromatography A, 1216(18), p.4125 - 4127, 2009/03
Times Cited Count:10 Percentile:30.80(Biochemical Research Methods)Dependence of Th recovery on HF concentration in nitric acid solutions (1 5 mol/dm
5 mol/dm ) containing 1
) containing 1 10
10 mol/dm
mol/dm of Th and various concentrations of HF was studied using a commercially available UTEVA resin column (for uranium and tetravalent actinide). Thorium recovery decreased with an increase in the HF concentration in the sample solutions. The concentration of HF at which Th recovery started to decrease was about 1
of Th and various concentrations of HF was studied using a commercially available UTEVA resin column (for uranium and tetravalent actinide). Thorium recovery decreased with an increase in the HF concentration in the sample solutions. The concentration of HF at which Th recovery started to decrease was about 1 10
10 mol/dm
mol/dm in 1 mol/dm
in 1 mol/dm HNO
HNO solution, about 1
solution, about 1 10
10 mol/dm
mol/dm in 3 mol/dm
in 3 mol/dm HNO
HNO solution, and about 1
solution, and about 1 10
10 mol/dm
mol/dm in 5 mol/dm
in 5 mol/dm HNO
HNO solution. When Al(NO
solution. When Al(NO )
) (0.2 mol/dm
(0.2 mol/dm ) or Fe(NO
) or Fe(NO )
) (0.6 mol/dm
(0.6 mol/dm ) was added as a masking agent for F
) was added as a masking agent for F into the Th solution containing 1
into the Th solution containing 1 10
10 mol/dm
mol/dm HF and 1 mol/dm
HF and 1 mol/dm HNO
HNO , the Th recovery improved from 1.4
, the Th recovery improved from 1.4 0.3% to 95
0.3% to 95 5% or 93
5% or 93 3%. Effective extraction of Th on UTEVA resin was achieved by selecting the concentration of HNO
3%. Effective extraction of Th on UTEVA resin was achieved by selecting the concentration of HNO and/or adding masking agents such as Al(NO
and/or adding masking agents such as Al(NO )
) according to the concentration of HF in the sample solution.
according to the concentration of HF in the sample solution.
Fujiwara, Asako; Kameo, Yutaka; Hoshi, Akiko; Haraga, Tomoko; Nakashima, Mikio
Journal of Chromatography A, 1140(1-2), p.163 - 167, 2007/01
Times Cited Count:25 Percentile:57.86(Biochemical Research Methods)Extraction chromatography with UTEVA resin was applied to separation of Th and U from control solutions prepared from a multi-element control solution and from sample solutions of solidified simulated waste. Thorium and U in control solutions with 1 to 5 M HNO were extracted with UTEVA resin and recovered with a solution containing 0.1 M HNO
were extracted with UTEVA resin and recovered with a solution containing 0.1 M HNO and 0.05 M oxalic acid to be separated from the other metallic elements. Extraction behavior of U in the sample solutions was similar to that in the control solutions, but extraction of Th was dependent on the concentration of HNO
and 0.05 M oxalic acid to be separated from the other metallic elements. Extraction behavior of U in the sample solutions was similar to that in the control solutions, but extraction of Th was dependent on the concentration of HNO . Thorium was extracted from 5 M HNO
. Thorium was extracted from 5 M HNO sample solutions but not from 1 M HNO
sample solutions but not from 1 M HNO sample solutions. We conjecture that thorium fluoride formation interferes with extraction of Th. Addition of Al(NO
sample solutions. We conjecture that thorium fluoride formation interferes with extraction of Th. Addition of Al(NO )
) and Fe(NO
and Fe(NO )
) , which have a higher stability constant with fluoride ion than Th does with it improved extractability of Th from 1 M HNO
, which have a higher stability constant with fluoride ion than Th does with it improved extractability of Th from 1 M HNO sample solution.
sample solution.
Asai, Shiho; Watanabe, Kazuo; Sugo, Takanobu*; Saito, Kyoichi*
Journal of Chromatography A, 1094(1-2), p.158 - 164, 2005/11
Times Cited Count:23 Percentile:54.55(Biochemical Research Methods)The analysis of radioactive species in radioactive wastes is essential to the safe and economical disposal of such wastes. Among radioactive species, alpha- and beta-emitting nuclides should be purified prior to various radiometric determinations. To overcome the disadvantages of the conventional separation techniques, we have proposed functional porous hollow-fiber membranes that achieve a high speed operation assisted by convective flow. Stable immobilization in aqueous media is ensured by the hydrophobic interaction between the hydrophobic moiety of the extractant and octadecyl part of octadecylamino group. In this study, HDEHP, which shows the selectivity for rare earth elements, such as yttrium, was immobilized onto the porous membrane. The amount of immobilized HDEHP increased with increasing molar conversion. This can be explained by the fact that an increase in the C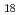 NH group allows the polymer brush to extend itself due to electrostatic repulsion originating from the amino part of the C
NH group allows the polymer brush to extend itself due to electrostatic repulsion originating from the amino part of the C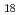 NH group.
NH group.
; Yoshida, Masaru; ; Omichi, Hideki;
Journal of Chromatography, 620, p.149 - 152, 1993/00
no abstracts in English
Yamanishi, Toshihiko; Kudo, Hiroshi
Journal of Chromatography, 475, p.125 - 134, 1989/00
Times Cited Count:9 Percentile:48.70(Chemistry, Analytical)no abstracts in English