Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 high energy resolution XAFS
high energy resolution XAFSYamamoto, Naoki*; Matsumura, Daiju; Hagihara, Yuto*; Tanaka, Kei*; Hasegawa, Yuta*; Ishii, Kenji*; Tanaka, Hirohisa*
Journal of Power Sources, 557, p.232508_1 - 232508_10, 2023/02
Times Cited Count:8 Percentile:49.56(Chemistry, Physical)Nasu, Mitsunori*; Yanai, Hiroshi*; Hirayama, Naoki*; Adachi, Hironori*; Kakizawa, Yu*; Shirase, Yuto*; Nishiyama, Hiromichi*; Kawamoto, Teppei*; Inukai, Junji*; Shinohara, Takenao; et al.
Journal of Power Sources, 530, p.231251_1 - 231251_11, 2022/05
Times Cited Count:32 Percentile:88.79(Chemistry, Physical) -LiV
-LiV O
O from polyoxovanadate cluster Li
from polyoxovanadate cluster Li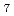 [V
[V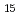 O
O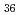 (CO
(CO )] as a high-performance cathode material and its reaction mechanism revealed by
)] as a high-performance cathode material and its reaction mechanism revealed by  XAFS
XAFSWang, H.*; Idobe, Jin*; Shimizu, Takeshi*; Matsumura, Daiju; Ina, Toshiaki*; Yoshikawa, Hirofumi*
Journal of Power Sources, 360, p.150 - 156, 2017/08
Times Cited Count:16 Percentile:43.93(Chemistry, Physical) and their Zr-O surface modified electrodes
and their Zr-O surface modified electrodesAbe, Machiko*; Iba, Hideki*; Suzuki, Kota*; Minamishima, Hiroaki*; Hirayama, Masaaki*; Tamura, Kazuhisa; Mizuki, Junichiro*; Saito, Tomohiro*; Ikuhara, Yuichi*; Kanno, Ryoji*
Journal of Power Sources, 345, p.108 - 119, 2017/03
Times Cited Count:12 Percentile:37.12(Chemistry, Physical)The surface structure of the Li(Ni, Co, Mn)O electrode was studied during charge/discharge process using electrochemical methods and X-ray/Neutron scattering techniques. It was found that during charge/discharge process the coverage of spinel structure increased. The spinel structure has low electrochemical activity and is not involved in Li insertion/extraction. After the surface modification, it was found that the coverage of the spinel structure did not increase. Further, it was also found out that the Li concentration at the electrode/electrolyte interface increased.
electrode was studied during charge/discharge process using electrochemical methods and X-ray/Neutron scattering techniques. It was found that during charge/discharge process the coverage of spinel structure increased. The spinel structure has low electrochemical activity and is not involved in Li insertion/extraction. After the surface modification, it was found that the coverage of the spinel structure did not increase. Further, it was also found out that the Li concentration at the electrode/electrolyte interface increased.
 surface under high voltage battery operation
surface under high voltage battery operationTaminato, So*; Hirayama, Masaaki*; Suzuki, Kota*; Tamura, Kazuhisa; Minato, Taketoshi*; Arai, Hajime*; Uchimoto, Yoshiharu*; Ogumi, Zempachi*; Kanno, Ryoji*
Journal of Power Sources, 307, p.599 - 603, 2016/03
Times Cited Count:36 Percentile:70.69(Chemistry, Physical)An epitaxial-film model electrode of LiCoO (104) was fabricated on SrRuO
(104) was fabricated on SrRuO (100)/Nb:SrTiO
(100)/Nb:SrTiO (100) using pulsed laser deposition. The 50 nm thick LiCoO
(100) using pulsed laser deposition. The 50 nm thick LiCoO (104) film exhibited lithium (de-)intercalation activity with a first discharge capacity of 119 mAh g
(104) film exhibited lithium (de-)intercalation activity with a first discharge capacity of 119 mAh g between 3.0 and 4.4 V, followed by a gradual capacity fading with subsequent charge-discharge cycles. In contrast, a 3.2 nm thick Li
between 3.0 and 4.4 V, followed by a gradual capacity fading with subsequent charge-discharge cycles. In contrast, a 3.2 nm thick Li PO
PO -coated film exhibited a higher intercalation capacity of 148 mAh g
-coated film exhibited a higher intercalation capacity of 148 mAh g with superior cycle retention than the uncoated film. In situ surface X-ray diffraction measurements revealed a small lattice change at the coated surface during the (de-)intercalation processes compared to the uncoated surface. The surface modification of LiCoO
with superior cycle retention than the uncoated film. In situ surface X-ray diffraction measurements revealed a small lattice change at the coated surface during the (de-)intercalation processes compared to the uncoated surface. The surface modification of LiCoO by the Li
by the Li PO
PO coating could lead to improvement of the structural stability at the surface region during lithium (de-)intercalation at high voltage.
coating could lead to improvement of the structural stability at the surface region during lithium (de-)intercalation at high voltage.
Takeda, Takeshi; Otsu, Iwao
Journal of Energy and Power Sources, 2(7), p.274 - 290, 2015/07
Sakamoto, Tomokazu*; Asazawa, Koichiro*; Martinez, U.*; Halevi, B.*; Suzuki, Toshiyuki*; Arai, Shigeo*; Matsumura, Daiju; Nishihata, Yasuo; Atanassov, P.*; Tanaka, Hirohisa*
Journal of Power Sources, 234, p.252 - 259, 2013/07
Times Cited Count:72 Percentile:86.37(Chemistry, Physical)Niwa, Hideharu*; Saito, Makoto*; Kobayashi, Masaki*; Harada, Yoshihisa*; Oshima, Masaharu*; Moriya, Shogo*; Matsubayashi, Katsuyuki*; Nabae, Yuta*; Kuroki, Shigeki*; Ikeda, Takashi; et al.
Journal of Power Sources, 223, p.30 - 35, 2013/02
Times Cited Count:18 Percentile:48.58(Chemistry, Physical)To design non-platinum, inexpensive, but high performance carbon-based cathode catalysts for polymer electrolyte fuel cells, it is important to elucidate the active site for oxygen reduction reaction (ORR). However, it is difficult to directly identify the active site by applying conventional structural or electronic probes to such complex systems. Here, we used C 1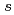 X-ray absorption spectroscopy (XAS) to observe electronic structure of carbon in iron phthalocyanine-based catalysts, and found a signature of edge exposure below the
X-ray absorption spectroscopy (XAS) to observe electronic structure of carbon in iron phthalocyanine-based catalysts, and found a signature of edge exposure below the 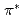 edge, whose intensity is well correlated with the ORR activity. These results demonstrate that C 1
edge, whose intensity is well correlated with the ORR activity. These results demonstrate that C 1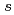 XAS can be used to characterize the ORR activity of carbon-based cathode catalysts in terms of the edge exposure.
XAS can be used to characterize the ORR activity of carbon-based cathode catalysts in terms of the edge exposure.
Kobayashi, Masaki*; Niwa, Hideharu*; Harada, Yoshihisa*; Horiba, Koji*; Oshima, Masaharu*; Ofuchi, Hironori*; Terakura, Kiyoyuki*; Ikeda, Takashi; Koshigoe, Yuka*; Ozaki, Junichi*; et al.
Journal of Power Sources, 196(20), p.8346 - 8351, 2011/10
Times Cited Count:32 Percentile:65.50(Chemistry, Physical)The electronic structure of Co atoms in CoPc-based carbon catalysts, which were prepared by pyrolyzing a mixture of CoPc and phenol resin polymer up to 1000 C, has been investigated using XAFS analysis and HXPES. The Co K XAFS spectra show that most of the Co atoms are in the metallic state and small quantities of oxidized Co components are present in the samples even after acid washing to remove Co atoms. Based on the difference in probing depth between XAFS and HXPES, it was found that after acid washing, the surface region with the aggregated Co clusters is primarily composed of metallic Co. Since the electrochemical properties remain almost unchanged even after the acid washing process, the residual metallic and oxidized Co atoms themselves will hardly contribute to the ORR activity of the CoPc-based carbon cathode catalysts, implying that the active sites of the CoPc-based catalysts primarily consist of light elements such as C and N.
C, has been investigated using XAFS analysis and HXPES. The Co K XAFS spectra show that most of the Co atoms are in the metallic state and small quantities of oxidized Co components are present in the samples even after acid washing to remove Co atoms. Based on the difference in probing depth between XAFS and HXPES, it was found that after acid washing, the surface region with the aggregated Co clusters is primarily composed of metallic Co. Since the electrochemical properties remain almost unchanged even after the acid washing process, the residual metallic and oxidized Co atoms themselves will hardly contribute to the ORR activity of the CoPc-based carbon cathode catalysts, implying that the active sites of the CoPc-based catalysts primarily consist of light elements such as C and N.
Niwa, Hideharu*; Kobayashi, Masaki*; Horiba, Koji*; Harada, Yoshihisa*; Oshima, Masaharu*; Terakura, Kiyoyuki*; Ikeda, Takashi; Koshigoe, Yuka*; Ozaki, Junichi*; Miyata, Seizo*; et al.
Journal of Power Sources, 196(3), p.1006 - 1011, 2011/02
Times Cited Count:93 Percentile:90.95(Chemistry, Physical)We report on the electronic structure of three different types of N-containing carbon-based cathode catalysts for polymer electrolyte fuel cells observed by hard X-ray photoemission spectroscopy. C 1s spectra show the importance of 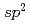 carbon network formation for the oxygen reduction reaction (ORR) activity. Samples having high oxygen reduction reaction activity in terms of oxygen reduction potential contain high concentration of graphite-like nitrogen. Based on a quantitative analysis of our results, the oxygen reduction reaction activity of the carbon-based cathode catalysts will be improved by increasing concentration of graphite-like nitrogen in a developed
carbon network formation for the oxygen reduction reaction (ORR) activity. Samples having high oxygen reduction reaction activity in terms of oxygen reduction potential contain high concentration of graphite-like nitrogen. Based on a quantitative analysis of our results, the oxygen reduction reaction activity of the carbon-based cathode catalysts will be improved by increasing concentration of graphite-like nitrogen in a developed 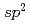 carbon network.
carbon network.
Yamaki, Tetsuya
Journal of Power Sources, 195(18), p.5848 - 5855, 2010/09
Times Cited Count:18 Percentile:47.28(Chemistry, Physical)Fuel-cell application requires that the electrolyte membrane should meet the characteristics; for examples, proton conductivity, mechanical strength, swelling properties, chemical stability and hydrogen/methanol permeability. When higher proton conductivity is pursued for practical applications, at least one of the other membrane properties is usually reduced; these trade-offs must be assessed in the current stage of technology. My talk reviews briefly original techniques of Japan Atomic Energy Agency, which should break the above trade-off relationship in the development of the membranes. The main topic will be systematic utilization of quantum beam technology covering (1) radiation crosslinking and graft polymerization by using  -ray and electron beam, (2) preparation of nano-structure controlled membranes by ion track technology, and (3) use of neutron scattering for membrane analysis.
-ray and electron beam, (2) preparation of nano-structure controlled membranes by ion track technology, and (3) use of neutron scattering for membrane analysis.
Niwa, Hideharu*; Horiba, Koji*; Harada, Yoshihisa*; Oshima, Masaharu*; Ikeda, Takashi; Terakura, Kiyoyuki*; Ozaki, Junichi*; Miyata, Seizo*
Journal of Power Sources, 187(1), p.93 - 97, 2009/02
Times Cited Count:445 Percentile:99.75(Chemistry, Physical)The electronic structure of nitrogens introduced in various carbon-based cathode catalysts for a polymer electrolyte fuel cell (PEFC) has been investigated using X-ray absorption spectroscopy (XAS). The profile of the 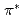 peaks at the pre-edge of the N 1s XAS spectra is used to determine the chemical states of nitrogens, which can be a marker of the oxygen reduction reaction (ORR) activity; it is found that catalysts that have relatively high amount of graphite-like nitrogen exhibit higher ORR activity than those having relatively high amount of pyridine-like nitrogen. We propose that effective doping of graphite-like nitrogen is a practical guideline for the synthesis of active carbon alloy catalysts.
peaks at the pre-edge of the N 1s XAS spectra is used to determine the chemical states of nitrogens, which can be a marker of the oxygen reduction reaction (ORR) activity; it is found that catalysts that have relatively high amount of graphite-like nitrogen exhibit higher ORR activity than those having relatively high amount of pyridine-like nitrogen. We propose that effective doping of graphite-like nitrogen is a practical guideline for the synthesis of active carbon alloy catalysts.
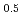 Mn
Mn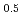 O
O
Sakamoto, Kazuyuki*; Konishi, Hiroaki*; Sonoyama, Noriyuki*; Yamada, Atsuo*; Tamura, Kazuhisa; Mizuki, Junichiro; Kanno, Ryoji*
Journal of Power Sources, 174(2), p.678 - 682, 2007/12
Times Cited Count:25 Percentile:59.38(Chemistry, Physical)Structure changes of LiNi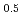 Mn
Mn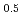 O
O were detected at the electrode/electrolyte interface of lithium cell using synchrotron X-ray scattering and two-dimensional model electrodes. The electrodes were constructed by an epitaxial film of LiNi
were detected at the electrode/electrolyte interface of lithium cell using synchrotron X-ray scattering and two-dimensional model electrodes. The electrodes were constructed by an epitaxial film of LiNi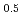 Mn
Mn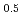 O
O synthesized by pulsed laser deposition (PLD) method. The orientation of the film depends on the substrate plane; the 2D layer of LiNi
synthesized by pulsed laser deposition (PLD) method. The orientation of the film depends on the substrate plane; the 2D layer of LiNi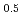 Mn
Mn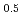 O
O is parallel to the SrTiO
is parallel to the SrTiO (1 1 0) substrate ((1 1 0) LiNi
(1 1 0) substrate ((1 1 0) LiNi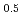 Mn
Mn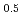 O
O //(1 1 0) SrTiO
//(1 1 0) SrTiO ), while the 2D layer is perpendicular to the SrTiO
), while the 2D layer is perpendicular to the SrTiO (1 1 1) substrate ((0 0 3) LiNi
(1 1 1) substrate ((0 0 3) LiNi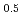 Mn
Mn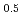 O
O //(1 1 1) SrTiO
//(1 1 1) SrTiO ). The
). The 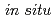 X-ray diffraction of LiNi
X-ray diffraction of LiNi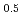 Mn
Mn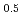 O
O (0 0 3) confirmed three-dimensional lithium diffusion through the two-dimensional transition meal layers. The intercalation reaction of LiNi
(0 0 3) confirmed three-dimensional lithium diffusion through the two-dimensional transition meal layers. The intercalation reaction of LiNi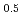 Mn
Mn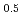 O
O will be discussed.
will be discussed.
 synchrotron X-ray reflectometry; A New experimental technique for LiCoO
synchrotron X-ray reflectometry; A New experimental technique for LiCoO model electrode
model electrodeHirayama, Masaaki*; Sonoyama, Noriyuki*; Abe, Takashi*; Minoura, Machiko*; Ito, Masumi*; Mori, Daisuke*; Yamada, Atsuo*; Kanno, Ryoji*; Terashima, Takahito*; Takano, Mikio*; et al.
Journal of Power Sources, 168(2), p.493 - 500, 2007/06
Times Cited Count:93 Percentile:90.14(Chemistry, Physical)A new experimental technique was developed for detecting structure changes at electrode/electrolyte interface of lithium cell using X-ray reflectometry and two-dimensional model electrodes with a restricted lattice-plane. The electrodes were constructed with an epitaxial film of LiCoO synthesized by pulsed laser deposition method. The anisotropic properties were confirmed by electrochemical measurements.
synthesized by pulsed laser deposition method. The anisotropic properties were confirmed by electrochemical measurements.  X-ray reflectivity measurements indicated that the impurity layer existed on the as-grown LiCoO
X-ray reflectivity measurements indicated that the impurity layer existed on the as-grown LiCoO was dissolved and a new SEI layer with lower density was formed after soaking into the electrolyte.
was dissolved and a new SEI layer with lower density was formed after soaking into the electrolyte.  X-ray reflectivity measurements indicated that the surface roughness of the intercalation (1 1 0) plane increased with applying voltages, while no significant changes in surface morphology were observed for the intercalation non-active (0 0 3) plane during the pristine stage of the charge-discharge process.
X-ray reflectivity measurements indicated that the surface roughness of the intercalation (1 1 0) plane increased with applying voltages, while no significant changes in surface morphology were observed for the intercalation non-active (0 0 3) plane during the pristine stage of the charge-discharge process.
Chen, J.; Asano, Masaharu; Yamaki, Tetsuya; Yoshida, Masaru
Journal of Power Sources, 158(1), p.69 - 77, 2006/07
Times Cited Count:101 Percentile:91.78(Chemistry, Physical)To develop a highly chemically stable polymer electrolyte membrane for application in a direct methanol fuel cell(DMFC), doubly crosslinked membranes were prepared by chemical crosslinking using bifunctional monomers, such as divinylbenzene(DVB) and bis(p, p-vinyl phenyl) ethane(BVPE), and by radiation crosslinking. The membranes were prepared by grafting of m, p-methylstyrene(MeSt) and p-tert-butylstyrene(tBuSt) into poly(ethylene-co-tetrafluoroethylene)(ETFE) films and subsequent sulfonation. The effects of the DVB and BVPE crosslinkers on the grafting kinetics and the properties of the prepared membranes, such as water uptake, proton conductivity and chemical stability were investigated. Radiation crosslinking was introduced by irradiation of the ETFE base film, the grafted film or the sulfonated membrane. The membrane crosslinked by DVB and BVPE crosslinkers and post-crosslinked by  -ray irradiation of the corresponding grafted film possessed the highest chemical stability among the prepared membranes, a significantly lower methanol permeability compared to Nafion membranes, and a better DMFC performance for high methanol feed concentration. Therefore, this doubly crosslinked membrane was promising for application in a DMFC where relatively high methanol concentration could be fed.
-ray irradiation of the corresponding grafted film possessed the highest chemical stability among the prepared membranes, a significantly lower methanol permeability compared to Nafion membranes, and a better DMFC performance for high methanol feed concentration. Therefore, this doubly crosslinked membrane was promising for application in a DMFC where relatively high methanol concentration could be fed.