Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ito, Kengo*; Takahashi, Shin*; Kato, Chizu*; Fukutani, Satoshi*; Matsumura, Tatsuro; Fujii, Toshiyuki*
Journal of Radioanalytical and Nuclear Chemistry, 334, p.2467 - 2475, 2025/02
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)In this study, the solvent extraction behavior of tin (Sn), specifically  Sn, from high-level radioactive waste was evaluated using six different extractants in a HNO
Sn, from high-level radioactive waste was evaluated using six different extractants in a HNO system. Among the tested extractants, N,N-Didodecyl-2-hydroxyacetoamide (HAA) exhibited higher efficiency, still not sufficient for industrial implementation. In systems where HCl was added to HNO
system. Among the tested extractants, N,N-Didodecyl-2-hydroxyacetoamide (HAA) exhibited higher efficiency, still not sufficient for industrial implementation. In systems where HCl was added to HNO , both tributyl phosphate (TBP) and N,N,N,N'-tetra-2-ethylhexyl diglycolamide (TEHDGA) achieved D
, both tributyl phosphate (TBP) and N,N,N,N'-tetra-2-ethylhexyl diglycolamide (TEHDGA) achieved D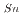 values greater than 1 at
values greater than 1 at  1 M HCl. However, due to practical challenges in industrial applications, HAA extraction in HNO
1 M HCl. However, due to practical challenges in industrial applications, HAA extraction in HNO systems, particularly at low Sn concentrations (0.0008 M), may provide a more effective solution for Sn recovery.
systems, particularly at low Sn concentrations (0.0008 M), may provide a more effective solution for Sn recovery.
 Cs/
Cs/ Cs isotope ratio
Cs isotope ratioShimada, Asako; Tsukahara, Takehiko*; Nomura, Masao*; Takeda, Seiji
Journal of Radioanalytical and Nuclear Chemistry, 333(12), p.6297 - 6310, 2024/12
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)Ito, Kengo*; Kawakami, Takahiro*; Kato, Chizu*; Fukutani, Satoshi*; Matsumura, Tatsuro; Fujii, Toshiyuki*
Journal of Radioanalytical and Nuclear Chemistry, 333(10), p.5183 - 5189, 2024/10
Times Cited Count:1 Percentile:57.00(Chemistry, Analytical)Solvent extraction behaviors of Se (VI) from nitric acid solutions were investigated with multiple extractants used for uranium, plutonium, minor actinides, and rare earthelements separation processes from high-level liquid waste. During the processes, Se remained in the residual aqueous solutions, as all extractants showed distributionratios  1. In contrast, Se showed distribution ratios
1. In contrast, Se showed distribution ratios  1 with
1 with 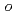 -phenylenediamine in dilute nitric acid (
-phenylenediamine in dilute nitric acid ( 2 M HNO
2 M HNO ), octanol as the organic phase, and concentrated nitric acid (8 M HNO
), octanol as the organic phase, and concentrated nitric acid (8 M HNO ) for back extraction, suggesting a potential new single separation process and recovery of Se.
) for back extraction, suggesting a potential new single separation process and recovery of Se.
Arai, Yoichi; Hasegawa, Kenta; Watanabe, So; Watanabe, Masayuki; Minowa, Kazuki*; Matsuura, Haruaki*; Hagura, Naoto*; Katsuki, Kenta*; Arai, Tsuyoshi*; Konishi, Yasuhiro*
Journal of Radioanalytical and Nuclear Chemistry, 333(7), p.3585 - 3593, 2024/07
Times Cited Count:1 Percentile:25.62(Chemistry, Analytical)Kimura, Yoshiki; Matsumoto, Tetsuya*; Yamaguchi, Tomoki
Journal of Radioanalytical and Nuclear Chemistry, 333(7), p.3541 - 3551, 2024/07
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)Ninomiya, Kazuhiko*; Kubo, Kenya*; Inagaki, Makoto*; Yoshida, Go*; Takeshita, Soshi*; Tampo, Motonobu*; Shimomura, Koichiro*; Kawamura, Naritoshi*; Strasser, P.*; Miyake, Yasuhiro*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 333(7), p.3445 - 3450, 2024/07
Times Cited Count:1 Percentile:57.00(Chemistry, Analytical)Hu, X.*; Fujita, Yoshitaka; Tsuchiya, Kunihiko; Fukutani, Satoshi*; Hori, Junichi*; Suzuki, Tatsuya*
Journal of Radioanalytical and Nuclear Chemistry, 333(11), p.6057 - 6063, 2024/05
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)no abstracts in English
 Cs contamination of Japanese mustard spinach by resuspended particles in areas with different contamination conditions
Cs contamination of Japanese mustard spinach by resuspended particles in areas with different contamination conditionsTatsuno, Takahiro*; Yoshimura, Kazuya; Nihei, Naoto*
Journal of Radioanalytical and Nuclear Chemistry, 333(3), p.1089 - 1096, 2024/03
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical) Ra purity as a target for
Ra purity as a target for  Ac production using a fast reactor
Ac production using a fast reactorSasaki, Yuto; Maeda, Shigetaka
Journal of Radioanalytical and Nuclear Chemistry, 333, p.5987 - 5996, 2024/02
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)Researchers are seeking alternative  Ac production methods because of the scarcity of
Ac production methods because of the scarcity of  Ac for targeted alpha therapy. Although
Ac for targeted alpha therapy. Although  Ra from waste sources has been considered, obtaining
Ra from waste sources has been considered, obtaining  Ra is challenging. Therefore, the authors focused on the contamination of minerals with
Ra is challenging. Therefore, the authors focused on the contamination of minerals with 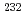 Th-derived
Th-derived 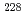 Ra while investigating methods to recover
Ra while investigating methods to recover  Ra from uranium ores. The effect of 228Ra contamination on
Ra from uranium ores. The effect of 228Ra contamination on  Ac production by
Ac production by  Ra transmutation using fast reactors was evaluated. Consequently, toxic
Ra transmutation using fast reactors was evaluated. Consequently, toxic 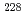 Ac contaminates
Ac contaminates  Ac. However, using the different half-lives of
Ac. However, using the different half-lives of  Ac and
Ac and 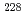 Ac, pure
Ac, pure  Ac can be obtained from the chemical separation of actinium after cooling for 5-8 days.
Ac can be obtained from the chemical separation of actinium after cooling for 5-8 days.
 Mo hot atoms made by a neutron capture method from
Mo hot atoms made by a neutron capture method from  -MoO
-MoO to water
to waterQuach, N. M.*; Ngo, M. C.*; Yang, Y.*; Nguyen, T. B.*; Nguyen, V. T.*; Fujita, Yoshitaka; Do, T. M. D.*; Nakayama, Tadachika*; Suzuki, Tatsuya*; Suematsu, Hisayuki*
Journal of Radioanalytical and Nuclear Chemistry, 332(10), p.4057 - 4064, 2023/10
Times Cited Count:4 Percentile:62.75(Chemistry, Analytical)Technetium-99m ( Tc) is the most widely used medical radioisotope in the world and is produced from molybdenum-99 (
Tc) is the most widely used medical radioisotope in the world and is produced from molybdenum-99 ( Mo). Production of
Mo). Production of  Mo via the neutron capture method draws attention as an alternative to fission-derived
Mo via the neutron capture method draws attention as an alternative to fission-derived  Mo due to non-proliferation issues, but the specific radioactivity of
Mo due to non-proliferation issues, but the specific radioactivity of  Mo is extremely low. In this work, a porous
Mo is extremely low. In this work, a porous  -MoO
-MoO wire was prepared as an irradiation target in order to improve the specific activity by extracting
wire was prepared as an irradiation target in order to improve the specific activity by extracting  Mo. Porous
Mo. Porous  -MoO
-MoO wire is synthesized from Mo metal wire by a two-step heating procedure. The hot atom effect of
wire is synthesized from Mo metal wire by a two-step heating procedure. The hot atom effect of  Mo was confirmed by activity and isotope measurements of the porous
Mo was confirmed by activity and isotope measurements of the porous  -MoO
-MoO wire after neutron irradiation and the water used for extraction. In term of the extraction effectiveness, the effectiveness of
wire after neutron irradiation and the water used for extraction. In term of the extraction effectiveness, the effectiveness of  Mo extraction in the porous
Mo extraction in the porous  -MoO
-MoO wire was comparable to that of commercial
wire was comparable to that of commercial  -MoO
-MoO powder.
powder.
Maeda, Makoto; Segawa, Mariko; Toh, Yosuke; Endo, Shunsuke; Nakamura, Shoji; Kimura, Atsushi
Journal of Radioanalytical and Nuclear Chemistry, 332(8), p.2995 - 2999, 2023/08
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical) Cs transfer from soils contaminated by resuspended particles to Japanese mustard spinach in difficult-to-return zone of Fukushima
Cs transfer from soils contaminated by resuspended particles to Japanese mustard spinach in difficult-to-return zone of FukushimaTatsuno, Takahiro*; Nihei, Naoto*; Yoshimura, Kazuya; Ote, Nobuhito*
Journal of Radioanalytical and Nuclear Chemistry, 332(6), p.1677 - 1686, 2023/06
Times Cited Count:1 Percentile:25.62(Chemistry, Analytical)Hidaka, Akihide
Journal of Radioanalytical and Nuclear Chemistry, 332(6), p.1607 - 1623, 2023/03
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)no abstracts in English
 (M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd
(M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd using cadmium chloride
using cadmium chlorideHayashi, Hirokazu; Shibata, Hiroki; Sato, Takumi; Otobe, Haruyoshi
Journal of Radioanalytical and Nuclear Chemistry, 332(2), p.503 - 510, 2023/02
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)The formation of MPd (M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu
(M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu -type GdPd
-type GdPd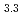 (
( = 0.4081
= 0.4081  0.0001 nm) and NpPd
0.0001 nm) and NpPd (
( = 0.4081
= 0.4081  0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi
0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi -type NpPd
-type NpPd . Chlorination of the MPd
. Chlorination of the MPd (M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl
(M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl formation.
formation.
 -ray analysis
-ray analysisKinoshita, Norikazu*; Noto, Takuma*; Nakajima, Hitoshi*; Kosako, Kazuaki*; Kato, Takahiro*; Kuroiwa, Yoichi*; Kurabe, Misako*; Sasaki, Yuki*; Torii, Kazuyuki*; Maeda, Makoto; et al.
Journal of Radioanalytical and Nuclear Chemistry, 332(2), p.479 - 486, 2023/02
Times Cited Count:2 Percentile:46.61(Chemistry, Analytical)Hagiwara, Hiroki; Kondo, Keietsu; Hidaka, Akihide
Journal of Radioanalytical and Nuclear Chemistry, 331(12), p.5905 - 5914, 2022/12
Times Cited Count:3 Percentile:42.88(Chemistry, Analytical) Sn in concrete rubble
Sn in concrete rubbleDo, V. K.; Furuse, Takahiro; Ota, Yuki; Iwahashi, Hiroyuki; Hirosawa, Takashi; Watanabe, Masahisa; Sato, Soichi
Journal of Radioanalytical and Nuclear Chemistry, 331(12), p.5631 - 5640, 2022/12
Times Cited Count:5 Percentile:53.26(Chemistry, Analytical) Sn is one of the long-lived fission products that might have been released into the environment after the Fukushima nuclear accident in Japan in 2011. The presence of radionuclides must be monitored for the proper treatment of wastes obtained from decommissioning accident-related nuclear facilities and the surrounding environment. In the work, we propose a reliable method for verifying the presence of
Sn is one of the long-lived fission products that might have been released into the environment after the Fukushima nuclear accident in Japan in 2011. The presence of radionuclides must be monitored for the proper treatment of wastes obtained from decommissioning accident-related nuclear facilities and the surrounding environment. In the work, we propose a reliable method for verifying the presence of  Sn in construction materials by combining the HCl-free solid phase extraction on TEVA resin and a selective measurement by inductively coupled plasma tandem mass spectrometry (ICP-MS/MS). The method has been optimized and characterized step by step. More than 95% of chemical recovery was achieved for Sn from typical concrete matrixes. The interference caused by an isobar
Sn in construction materials by combining the HCl-free solid phase extraction on TEVA resin and a selective measurement by inductively coupled plasma tandem mass spectrometry (ICP-MS/MS). The method has been optimized and characterized step by step. More than 95% of chemical recovery was achieved for Sn from typical concrete matrixes. The interference caused by an isobar  Te and possible polyatomic interferences from matrixes were effectively suppressed by the developed chemical separation and the tandem MS/MS configuration. The total decontamination factor for the Te interference was of the order of 10
Te and possible polyatomic interferences from matrixes were effectively suppressed by the developed chemical separation and the tandem MS/MS configuration. The total decontamination factor for the Te interference was of the order of 10 . The estimated method detection limit for
. The estimated method detection limit for  Sn in concrete as measured at m/z = 160 was 12.1 pg g
Sn in concrete as measured at m/z = 160 was 12.1 pg g , which is equivalent to 6.1 mBq g
, which is equivalent to 6.1 mBq g .
.
Akuzawa, Tadashi*; Kim, S.-Y.*; Kubota, Masahiko*; Wu, H.*; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki; Arai, Tsuyoshi*
Journal of Radioanalytical and Nuclear Chemistry, 331(12), p.5851 - 5858, 2022/12
Times Cited Count:5 Percentile:61.74(Chemistry, Analytical)Liu, J.; Dotsuta, Yuma; Sumita, Takehiro; Kitagaki, Toru; Onuki, Toshihiko; Kozai, Naofumi
Journal of Radioanalytical and Nuclear Chemistry, 331(6), p.2785 - 2794, 2022/06
Times Cited Count:4 Percentile:53.26(Chemistry, Analytical)Remnant nuclear fuel debris in the damaged nuclear reactors at the Fukushima Daiichi Nuclear Power Plant (FDNPP) has contacted the groundwater containing microorganisms for over ten years. Herein, we report the possibility of bacterial alteration of fuel debris. We investigated the physical and chemical changes of fuel debris simulants (FDS) in the powder and pellet forms via exposure to two ubiquitous bacteria, Pseudomonas fluorescens and Bacillus subtilis. In the experiments using FDS composed of the powders of Fe(0), solid solution of CeO and ZrO
and ZrO , and SiO
, and SiO , Ce, Zr, and Si were hardly dissolved, while Fe was dissolved, a fraction of the dissolved Fe was present in the liquid phase as Fe(II) and Fe(III), and the rest was precipitated as the nano-sized particles of iron (hydr)oxides. In the experiment using P. fluorescens and FDS pellet pieces prepared by melting the Fe(0) particles and solid solution of CeO
, Ce, Zr, and Si were hardly dissolved, while Fe was dissolved, a fraction of the dissolved Fe was present in the liquid phase as Fe(II) and Fe(III), and the rest was precipitated as the nano-sized particles of iron (hydr)oxides. In the experiment using P. fluorescens and FDS pellet pieces prepared by melting the Fe(0) particles and solid solution of CeO and ZrO
and ZrO , the bacteria selectively gathered on the Fe(0) particle surface and made corrosion pits. These results suggest that bacteria in groundwater corrode the iron in fuel debris at FDNPP, change fuel debris into porous one, releasing the nano-sized iron (hydr)oxide particles into the water.
, the bacteria selectively gathered on the Fe(0) particle surface and made corrosion pits. These results suggest that bacteria in groundwater corrode the iron in fuel debris at FDNPP, change fuel debris into porous one, releasing the nano-sized iron (hydr)oxide particles into the water.
Nomizu, Daiki; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Katsuta, Shoichi*
Journal of Radioanalytical and Nuclear Chemistry, 331(3), p.1483 - 1493, 2022/03
Times Cited Count:7 Percentile:74.40(Chemistry, Analytical)We studied the successive formation of water soluble DGA (diglycolamide) and DOODA (dioxaoctanediamide) for the mutual separation of Ln in this extraction system. TODGA (tetraoctyl-diglycolamide) and DOODA(C8) (tetraoctyl-dioxaoctanediamide) have the opposite trend to extract light and heavy Ln through Ln-patterns. Metal-complexes of two folding Ln ions with water-soluble DOODA and three folding with DGA are found and their observed formation constants are calculated. The suitable separation condition (aqueous phase: 30 mM DOODA(C2) in 1 M nitric acid, organic phase: 0.1 M TODGA in n-dodecane) of multi-stage extraction (10  10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.