Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Onutai, S.; Sato, Junya; Osugi, Takeshi
Journal of Solid State Chemistry, 319, p.123808_1 - 123808_10, 2023/03
Times Cited Count:25 Percentile:97.70(Chemistry, Inorganic & Nuclear) F and comparison of the structure among perovskite-type oxyfluorides
F and comparison of the structure among perovskite-type oxyfluoridesKatsumata, Tetsuhiro*; Suzuki, Ryo*; Sato, Naoto*; Suzuki, Shumpei*; Nakashima, Mamoru*; Inaguma, Yoshiyuki*; Mori, Daisuke*; Aimi, Akihisa*; Yoneda, Yasuhiro
Journal of Solid State Chemistry, 279, p.120919_1 - 120919_8, 2019/11
Times Cited Count:12 Percentile:56.06(Chemistry, Inorganic & Nuclear)Wang, H.*; Idobe, Jin*; Matsumura, Daiju; Yoshikawa, Hirofumi*
Journal of Solid State Electrochemistry, 22(7), p.2067 - 2071, 2018/07
Times Cited Count:19 Percentile:42.67(Electrochemistry) Ni
Ni Mn
Mn Co
Co O
O in a lithium-ion battery
in a lithium-ion batteryKonishi, Hiroaki*; Hirano, Tatsumi*; Takamatsu, Daiko*; Gunji, Akira*; Feng, X.*; Furutsuki, Sho*; Okumura, Takafumi*; Terada, Shohei*; Tamura, Kazuhisa
Journal of Solid State Chemistry, 262, p.294 - 300, 2018/06
Times Cited Count:10 Percentile:47.54(Chemistry, Inorganic & Nuclear)The potential in each state of charge (SOC) during charging of Li Ni
Ni Mn
Mn Co
Co O
O is higher than that during discharging. To clarify the effect of chargedischarge operating conditions on the electrochemical reaction, Li
is higher than that during discharging. To clarify the effect of chargedischarge operating conditions on the electrochemical reaction, Li Ni
Ni Mn
Mn Co
Co O
O was charged and discharged under various charge-discharge operating ranges, and OCP, crystal structure, and oxidation states of the ransition metals were evaluated by electrochemical measurement, XRD, and XAFS. These results indicate that OCP, lattice parameters, and oxidation states of the transition metals of Li
was charged and discharged under various charge-discharge operating ranges, and OCP, crystal structure, and oxidation states of the ransition metals were evaluated by electrochemical measurement, XRD, and XAFS. These results indicate that OCP, lattice parameters, and oxidation states of the transition metals of Li Ni
Ni Mn
Mn Co
Co O
O in each SOC are not constant. The XRD results indicate that two phases, namely, LiNi
in each SOC are not constant. The XRD results indicate that two phases, namely, LiNi Mn
Mn Co
Co O
O -like and Li
-like and Li MnO
MnO -like, exist in Li
-like, exist in Li Ni
Ni Mn
Mn Co
Co O
O .
.
 Ni
Ni Mn
Mn Co
Co O
O used for lithium ion batteries
used for lithium ion batteriesKonishi, Hiroaki*; Hirano, Tatsumi*; Takamatsu, Daiko*; Gunji, Akira*; Feng, X.*; Furutsuki, Sho*; Okumura, Takafumi*; Terada, Shohei*; Tamura, Kazuhisa
Journal of Solid State Chemistry, 258, p.225 - 231, 2018/02
Times Cited Count:9 Percentile:43.89(Chemistry, Inorganic & Nuclear)Li Ni
Ni Mn
Mn Co
Co O
O is known as one of the cathode electrode material for Li ion batteries and its structure during charge and discharge process was investigated using electrochemical method and X-ray diffraction. It was found that in the charge process the structure changes in the order of Li
is known as one of the cathode electrode material for Li ion batteries and its structure during charge and discharge process was investigated using electrochemical method and X-ray diffraction. It was found that in the charge process the structure changes in the order of Li MnO
MnO , LiNi
, LiNi Mn
Mn Co
Co O
O , and Li
, and Li MnO
MnO . On the other hand, in the discharge process, the structure changes in the order of Li
. On the other hand, in the discharge process, the structure changes in the order of Li MnO
MnO and LiNi
and LiNi Mn
Mn Co
Co O
O .
.
Iizuka, Riko*; Komatsu, Kazuki*; Kagi, Hiroyuki*; Nagai, Takaya*; Sano, Asami; Hattori, Takanori; Goto, Hirotada*; Yagi, Takehiko*
Journal of Solid State Chemistry, 218, p.95 - 102, 2014/10
Times Cited Count:7 Percentile:31.69(Chemistry, Inorganic & Nuclear)In situ neutron diffraction measurements combined with the pulsed neutron source at the Japan Proton Accelerator Research Complex (J-PARC) were conducted on high-pressure polymorphs of deuterated portlandite (Ca(OD) ) using a Paris-Edinburgh cell and a multi-anvil press. The atomic positions including hydrogen for the unquenchable high-pressure phase at room temperature (phase II') were first clarified. The bent hydrogen bonds under high pressure were consistent with results from Raman spectroscopy. The structure of the high-pressure and high-temperature phase (Phase II) was concordant with that observed previously by another group for a recovered sample. The observations elucidate the phase transition mechanism among the polymorphs, which involves the sliding of CaO polyhedral layers, position modulations of Ca atoms, and recombination of Ca-O bonds accompanied by the reorientation of hydrogen to form more stable hydrogen bonds.
) using a Paris-Edinburgh cell and a multi-anvil press. The atomic positions including hydrogen for the unquenchable high-pressure phase at room temperature (phase II') were first clarified. The bent hydrogen bonds under high pressure were consistent with results from Raman spectroscopy. The structure of the high-pressure and high-temperature phase (Phase II) was concordant with that observed previously by another group for a recovered sample. The observations elucidate the phase transition mechanism among the polymorphs, which involves the sliding of CaO polyhedral layers, position modulations of Ca atoms, and recombination of Ca-O bonds accompanied by the reorientation of hydrogen to form more stable hydrogen bonds.
 In
In O
O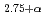
Nagasaki, Takanori*; Shiotani, Shinya*; Igawa, Naoki; Yoshino, Masahito*; Iwasaki, Kota*; Fukazawa, Hiroshi; Utsumi, Wataru
Journal of Solid State Chemistry, 182(10), p.2632 - 2639, 2009/10
Times Cited Count:8 Percentile:29.98(Chemistry, Inorganic & Nuclear)We propose a new method, a difference maximum entropy method (MEM) analysis of the neutron diffraction data, for revealing the detailed structure around hydrogen atoms in proton-conducting oxides. This MEM analysis uses the differences between the structure factors of protium- and deuterium-dissolved crystals. Simulations demonstrate that it not only provides the distribution of hydrogen atoms alone, but also improves the spatial resolution of MEM mapping around hydrogen atoms. Applied to actual diffraction data of protium- and deuterium-dissolved BaSn In
In O
O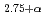 at 9 K, difference MEM analysis reveals that O-D bonds mostly tilt towards the second nearest oxygen atoms, and that the distributions of deuterium and oxygen atoms are probably insignificant in interstitial regions.
at 9 K, difference MEM analysis reveals that O-D bonds mostly tilt towards the second nearest oxygen atoms, and that the distributions of deuterium and oxygen atoms are probably insignificant in interstitial regions.
Kondo, Toshihiro*; Tamura, Kazuhisa; Takakusagi, Satoru*; Kitamura, Ken*; Takahashi, Masamitsu; Mizuki, Junichiro; Uosaki, Kohei*
Journal of Solid State Electrochemistry, 13(7), p.1141 - 1145, 2009/07
Times Cited Count:6 Percentile:14.35(Electrochemistry)The interfacial structures of Ag bilayer prepared by underpotential deposition on Au(111) were determined by STM and SXS before and after oxidative adsorption and after desorption of a SAM of C SH in alkaline ethanol solution. While no structural change was observed after oxidative formation of C
SH in alkaline ethanol solution. While no structural change was observed after oxidative formation of C SH SAM on the Ag/Au(111) in an ethanol solution containing 20 mM KOH and 0.1 mM C
SH SAM on the Ag/Au(111) in an ethanol solution containing 20 mM KOH and 0.1 mM C SH, some of the Ag atoms in the bilayer were stripped when the SAM was reductively desorbed.
SH, some of the Ag atoms in the bilayer were stripped when the SAM was reductively desorbed.
 Mn
Mn O
O (0
(0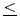 x
x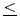 1)
1)Yoshii, Kenji; Ikeda, Naoshi*; Michiuchi, Takamasa*; Yokota, Yusuke*; Okajima, Yuka; Yoneda, Yasuhiro; Matsuo, Yoji*; Horibe, Yoichi*; Mori, Shigeo*
Journal of Solid State Chemistry, 182(7), p.1611 - 1618, 2009/06
Times Cited Count:15 Percentile:50.11(Chemistry, Inorganic & Nuclear)We have investigated the magnetic and dielectric properties of YbFe Mn
Mn O
O (0
(0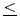 x
x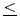 1), which is an Fe-site-substituted system of new multiferroic oxides RFe
1), which is an Fe-site-substituted system of new multiferroic oxides RFe O
O (R=Y, Ho-Lu). X-ray diffraction measurements show that a solid solution is formed between YbFe
(R=Y, Ho-Lu). X-ray diffraction measurements show that a solid solution is formed between YbFe O
O (x=0) and YbFeMnO
(x=0) and YbFeMnO (x=1) for 0
(x=1) for 0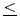 x
x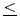 1. The valence of the Mn ion is determined to be
1. The valence of the Mn ion is determined to be  2+, consistently with the suppression of low-temperature magnetization by the Mn substitution. The magnetic transition temperature and the dielectric constant decrease monotonically with increasing x. This result plausibly confirms that the magnetic and dielectric properties in oxides isostructural with RFe
2+, consistently with the suppression of low-temperature magnetization by the Mn substitution. The magnetic transition temperature and the dielectric constant decrease monotonically with increasing x. This result plausibly confirms that the magnetic and dielectric properties in oxides isostructural with RFe O
O are governed by the electron transfer between Fe-site ions, unlike ordinary ferroelectrics and dielectrics, in which the ionic displacement plays a key role. The possibility for application is briefly discussed.
are governed by the electron transfer between Fe-site ions, unlike ordinary ferroelectrics and dielectrics, in which the ionic displacement plays a key role. The possibility for application is briefly discussed.
 -type montmorillonite; Potential chemical species of iron contaminant
-type montmorillonite; Potential chemical species of iron contaminantKozai, Naofumi; Inada, Koichi*; Adachi, Yoshifusa*; Kawamura, Sachi*; Kashimoto, Yusuke*; Kozaki, Tamotsu*; Sato, Seichi*; Onuki, Toshihiko; Sakai, Takuro; Sato, Takahiro; et al.
Journal of Solid State Chemistry, 180(8), p.2279 - 2289, 2007/08
Times Cited Count:14 Percentile:47.11(Chemistry, Inorganic & Nuclear)Fe -montmorillonite with Fe
-montmorillonite with Fe ions occupying cation exchange sites is an ideal transformation product in bentonite buffer material. We previously prepared a Fe
ions occupying cation exchange sites is an ideal transformation product in bentonite buffer material. We previously prepared a Fe -montmorillonite sample using a FeCl
-montmorillonite sample using a FeCl solution under an inert gas condition. This study attempted to determine the potential contaminant iron chemical species in the sample. It was found that a small amount of Cl
solution under an inert gas condition. This study attempted to determine the potential contaminant iron chemical species in the sample. It was found that a small amount of Cl ions remained dispersed throughout the sample. The Cl
ions remained dispersed throughout the sample. The Cl ion retention may be due to the adsorption of FeCl
ion retention may be due to the adsorption of FeCl in the initial FeCl
in the initial FeCl solution and the subsequent containment of the Cl
solution and the subsequent containment of the Cl ions that are dissociated from the FeCl
ions that are dissociated from the FeCl during excess salt removal treatment. The latter may be explained by the slow release of the remaining Cl
during excess salt removal treatment. The latter may be explained by the slow release of the remaining Cl ions from the collapsed interlayer of the montmorillonite or the transformation of a minor fraction of the remaining FeCl
ions from the collapsed interlayer of the montmorillonite or the transformation of a minor fraction of the remaining FeCl to iron (III) hydroxide chloride complexes having low solubility.
to iron (III) hydroxide chloride complexes having low solubility.
 )
) and CsFe(SO
and CsFe(SO )
)
Inami, Toshiya
Journal of Solid State Chemistry, 180(7), p.2075 - 2079, 2007/07
Times Cited Count:34 Percentile:77.17(Chemistry, Inorganic & Nuclear)Powder specimens of the layered triangular lattice antiferromagnets RbFe(MoO )
) and CsFe(SO
and CsFe(SO )
) were prepared and neutron powder diffraction experiments were carried out in order to determine the magnetic structure. Magnetic structure of both compounds is so-called 120
were prepared and neutron powder diffraction experiments were carried out in order to determine the magnetic structure. Magnetic structure of both compounds is so-called 120 structure in the triangular plane and incommensurate between the plane. The ordered moments are confined in the basal
structure in the triangular plane and incommensurate between the plane. The ordered moments are confined in the basal 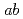 -plane. It is also found that RbFe(MoO
-plane. It is also found that RbFe(MoO )
) shows structural phase transition at around 190 K from
shows structural phase transition at around 190 K from 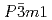 to
to 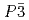 .
.
 Pb
Pb O
O
Yoshii, Kenji; Mizumaki, Masaichiro*; Kato, Kazuo*; Uruga, Tomoya*; Abe, Hideki*; Nakamura, Akio; Shimojo, Yutaka; Ishii, Yoshinobu; Morii, Yukio
Journal of Solid State Chemistry, 180(1), p.377 - 381, 2007/01
Times Cited Count:6 Percentile:23.11(Chemistry, Inorganic & Nuclear)no abstracts in English
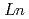
 Ca
Ca MnO
MnO (
(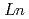 =Ho, Er, Tm, Yb and Lu)
=Ho, Er, Tm, Yb and Lu)Yoshii, Kenji; Abe, Hideki*; Ikeda, Naoshi*
Journal of Solid State Chemistry, 178(12), p.3615 - 3623, 2005/12
Times Cited Count:26 Percentile:67.78(Chemistry, Inorganic & Nuclear)no abstracts in English
 O
O -ZrO
-ZrO system
systemWang, J.*; Otobe, Haruyoshi; Nakamura, Akio; Takeda, Masuo*
Journal of Solid State Chemistry, 176(1), p.105 - 110, 2003/11
Times Cited Count:14 Percentile:43.66(Chemistry, Inorganic & Nuclear)no abstracts in English
Mizumaki, Masaichiro*; Yoshii, Kenji; Kitazawa, Hideaki*; Tanida, Hajime*
Journal of Solid State Chemistry, 171(1-2), p.291 - 294, 2003/02
Times Cited Count:5 Percentile:17.13(Chemistry, Inorganic & Nuclear)no abstracts in English
 Dy
Dy TiO
TiO
Yoshii, Kenji; Mizumaki, Masaichiro*; Nakamura, Akio; Abe, Hideki*
Journal of Solid State Chemistry, 171(1-2), p.345 - 348, 2003/02
Times Cited Count:9 Percentile:30.15(Chemistry, Inorganic & Nuclear)no abstracts in English
 ErRuO
ErRuO
Izumiyama, Yuki*; Doi, Yoshihiro*; Wakeshima, Makoto*; Hinatsu, Yukio*; Nakamura, Akio; Ishii, Yoshinobu
Journal of Solid State Chemistry, 169(1), p.125 - 130, 2002/11
Times Cited Count:42 Percentile:81.90(Chemistry, Inorganic & Nuclear)no abstracts in English
Abe, Hideki*; Yoshii, Kenji
Journal of Solid State Chemistry, 165(2), p.372 - 374, 2002/05
Times Cited Count:8 Percentile:29.25(Chemistry, Inorganic & Nuclear)no abstracts in English
 (Ln=Ho, Er, Tm, Yb and Lu)
(Ln=Ho, Er, Tm, Yb and Lu)Yoshii, Kenji; Abe, Hideki*
Journal of Solid State Chemistry, 165(1), p.131 - 135, 2002/04
Times Cited Count:47 Percentile:84.67(Chemistry, Inorganic & Nuclear)no abstracts in English
 Pr
Pr CrO
CrO
Yoshii, Kenji; Nakamura, Akio; Ishii, Yoshinobu; Morii, Yukio
Journal of Solid State Chemistry, 162(1), p.84 - 89, 2001/11
Times Cited Count:105 Percentile:96.62(Chemistry, Inorganic & Nuclear)no abstracts in English