Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Li, F.*; Tang, X.*; Fei, Y.*; Zhang, J.*; Liu, J.*; Lang, P.*; Che, G.*; Zhao, Z.*; Zheng, Y.*; Fang, Y.*; et al.
Journal of the American Chemical Society, 147(17), p.14054 - 14059, 2025/04
Times Cited Count:1 Percentile:0.00(Chemistry, Multidisciplinary)We synthesized a crystalline graphane nanoribbon (GANR) via pressure-induced polymerization of 2,2'-bipyrazine (BPZ). By performing Rietveld refinement of in situ neutron diffraction data, nuclear magnetic resonance spectroscopy, infrared spectra, and theoretical calculation, we found that BPZ experienced Diels-Alder polymerization between the 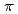
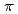 stacked aromatic rings, and formed extended boat-GANR structures with exceptional long-range order. The unreacted -C=N- groups bridge the two ends of the boat, and ready for further functionalization. The GANR has a bandgap of 2.25 eV, with booming photoelectric response (
stacked aromatic rings, and formed extended boat-GANR structures with exceptional long-range order. The unreacted -C=N- groups bridge the two ends of the boat, and ready for further functionalization. The GANR has a bandgap of 2.25 eV, with booming photoelectric response (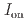 /
/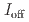 =18.8). Our work highlights that the high-pressure topochemical polymerization is a promising method for the precise synthesis of graphane with specific structure and desired properties.
=18.8). Our work highlights that the high-pressure topochemical polymerization is a promising method for the precise synthesis of graphane with specific structure and desired properties.
Ling, B.-K.*; Chang, M.*; Zhai, Y.-Q.*; Deng, J.*; Kofu, Maiko*; Guo, H.*; Zhao, J.*; Fu, Z.*; Zheng, Y.-Z.*
Journal of the American Chemical Society, 147(13), p.10935 - 10942, 2025/03
Times Cited Count:1 Percentile:83.04(Chemistry, Multidisciplinary)Horiike, Yuki*; Aoki, Hiroyuki; Ouchi, Makoto*; Terashima, Takaya*
Journal of the American Chemical Society, 147(8), p.6727 - 6738, 2025/02
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)
Lim, G.-C.*; Fukutani, Katsuyuki; 8 of others*
Journal of the American Chemical Society, 146(46), p.32013 - 32021, 2024/11
Times Cited Count:3 Percentile:51.94(Chemistry, Multidisciplinary)Nagai, Takayuki*; Hagihara, Masato; Yokoi, Rie*; Moriwake, Hiroki*; Kimura, Tsuyoshi*
Journal of the American Chemical Society, 146(33), p.23348 - 23355, 2024/08
Times Cited Count:1 Percentile:31.48(Chemistry, Multidisciplinary)Nakanishi, Takumi*; Hori, Yuta*; Shigeta, Yasuteru*; Sato, Hiroyasu*; Kiyanagi, Ryoji; Munakata, Koji*; Ohara, Takashi; Okazawa, Atsushi*; Shimada, Rintaro*; Sakamoto, Akira*; et al.
Journal of the American Chemical Society, 145(35), p.19177 - 19181, 2023/08
Times Cited Count:5 Percentile:49.39(Chemistry, Multidisciplinary)Zhang, P.*; Tang, X.*; Wang, Y.*; Wang, X.*; Gao, D.*; Li, Y.*; Zheng, H.*; Wang, Y.*; Wang, X.*; Fu, R.*; et al.
Journal of the American Chemical Society, 142(41), p.17662 - 17669, 2020/10
Times Cited Count:34 Percentile:78.58(Chemistry, Multidisciplinary)Solid-state topochemical polymerization (SSTP) is a promising method to construct functional crystalline polymeric materials, but in contrast to various reactions that happen in solution, only very limited types of SSTP reactions are reported. Diels-Alder (DA) and dehydro-DA (DDA) reactions are textbook reactions for preparing six-membered rings in solution but are scarcely seen in solid-state synthesis. Here, using multiple cutting-edge techniques, we demonstrate that the solid 1,4-diphenylbutadiyne (DPB) undergoes a DDA reaction under 10-20 GPa with the phenyl as the dienophile. The crystal structure at the critical pressure shows that this reaction is "distance-selected". The distance of 3.2 between the phenyl and the phenylethynyl facilitates the DDA reaction, while the distances for other DDA and 1,4-addition reactions are too large to allow the bonding. The obtained products are crystalline armchair graphitic nanoribbons, and hence our studies open a new route to construct the crystalline carbon materials with atomic-scale control.
between the phenyl and the phenylethynyl facilitates the DDA reaction, while the distances for other DDA and 1,4-addition reactions are too large to allow the bonding. The obtained products are crystalline armchair graphitic nanoribbons, and hence our studies open a new route to construct the crystalline carbon materials with atomic-scale control.
Sato, Tetsuya; Asai, Masato; Borschevsky, A.*; Beerwerth, R.*; Kaneya, Yusuke*; Makii, Hiroyuki; Mitsukai, Akina*; Nagame, Yuichiro; Osa, Akihiko; Toyoshima, Atsushi; et al.
Journal of the American Chemical Society, 140(44), p.14609 - 14613, 2018/11
Times Cited Count:32 Percentile:69.40(Chemistry, Multidisciplinary)The first ionization potential (IP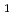 ) yields information on valence electronic structure of an atom. IP
) yields information on valence electronic structure of an atom. IP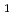 values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP
values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP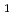 of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP
of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP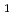 among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP
among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP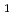 values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP
values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP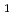 value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
Inukai, Munehiro*; Horike, Satoshi*; Itakura, Tomoya*; Shinozaki, Ryota*; Ogiwara, Naoki*; Umeyama, Daiki*; Nagarker, S.*; Nishiyama, Yusuke*; Malon, M.*; Hayashi, Akari*; et al.
Journal of the American Chemical Society, 138(27), p.8505 - 8511, 2016/07
Times Cited Count:152 Percentile:95.40(Chemistry, Multidisciplinary) as the origin of volume collapse
as the origin of volume collapseYu, R.*; Hojo, Hajime*; Watanuki, Tetsu; Mizumaki, Masaichiro*; Mizokawa, Takashi*; Oka, Kengo*; Kim, H.*; Machida, Akihiko; Sakaki, Koji*; Nakamura, Yumiko*; et al.
Journal of the American Chemical Society, 137(39), p.12719 - 12728, 2015/10
Times Cited Count:40 Percentile:72.17(Chemistry, Multidisciplinary)no abstracts in English
Unno, Masayoshi*; Ishikawa, Kumiko*; Kusaka, Katsuhiro*; Tamada, Taro; Hagiwara, Yoshinori*; Sugishima, Masakazu*; Wada, Kei*; Yamada, Taro*; Tomoyori, Katsuaki; Hosoya, Takaaki*; et al.
Journal of the American Chemical Society, 137(16), p.5452 - 5460, 2015/04
Times Cited Count:29 Percentile:62.63(Chemistry, Multidisciplinary)Phycocyanobilin, a light-harvesting and photoreceptor pigment in higher plants, algae, and cyanobacteria, is synthesized from biliverdin IX (BV) by phycocyanobilin:ferredoxin oxidoreductase (PcyA) via two steps of two-proton-coupled two-electron reduction. We determined the neutron structure of PcyA from cyanobacteria complexed with BV, revealing the exact location of the hydrogen atoms involved in catalysis. Notably, approximately half of the BV bound to PcyA was BVH
(BV) by phycocyanobilin:ferredoxin oxidoreductase (PcyA) via two steps of two-proton-coupled two-electron reduction. We determined the neutron structure of PcyA from cyanobacteria complexed with BV, revealing the exact location of the hydrogen atoms involved in catalysis. Notably, approximately half of the BV bound to PcyA was BVH , a state in which all four pyrrole nitrogen atoms were protonated. The protonation states of BV complemented the protonation of adjacent Asp105. The "axial "water molecule that interacts with the neutral pyrrole nitrogen of the A-ring was identified. His88 N
, a state in which all four pyrrole nitrogen atoms were protonated. The protonation states of BV complemented the protonation of adjacent Asp105. The "axial "water molecule that interacts with the neutral pyrrole nitrogen of the A-ring was identified. His88 N was protonated to form a hydrogen bond with the lactam O atom of the BV A-ring. His88 and His74 were linked by hydrogen bonds via H
was protonated to form a hydrogen bond with the lactam O atom of the BV A-ring. His88 and His74 were linked by hydrogen bonds via H O
O . These results imply that Asp105, His88, and the axial water molecule contribute to proton transfer during PcyA catalysis.
. These results imply that Asp105, His88, and the axial water molecule contribute to proton transfer during PcyA catalysis.
Chai, G.-L.*; Hou, Z.*; Shu, D.-J.*; Ikeda, Takashi; Terakura, Kiyoyuki*
Journal of the American Chemical Society, 136(39), p.13629 - 13640, 2014/10
Times Cited Count:281 Percentile:97.77(Chemistry, Multidisciplinary)Carbon alloy catalysts (CACs) are promising catalysts for oxygen reduction reaction (ORR) to substitute Pt. However, despite extensive studies on CACs the reaction sites and mechanisms for ORR are still in controversy. Herein, we present rather general consideration on possible ORR mechanisms for various structures in nitrogen doped CACs based on the first principles calculations. Our study indicates that only a particular structure of a nitrogen pair doped Stone-Wales defect provides a good active site. The ORR activity of this structure can be tuned by the curvature around the active site, which makes its limiting potential approaching the maximum limiting potential (0.80 V) in the volcano plot for the ORR activity of CACs. The calculated results can be compared with the recent experimental ones of the half wave potential for CAC systems that range from 0.60 V to 0.80 V in the reversible-hydrogen-electrode scale.
 with perovskite- and LiNbO
with perovskite- and LiNbO -type structures
-type structuresInaguma, Yoshiyuki*; Tanaka, Kie*; Tsuchiya, Takeshi*; Mori, Daisuke*; Katsumata, Tetsuhiro*; Oba, Tomonori*; Hiraki, Koichi*; Takahashi, Toshihiro*; Saito, Hiroyuki
Journal of the American Chemical Society, 133(42), p.16920 - 16929, 2011/09
Times Cited Count:98 Percentile:87.34(Chemistry, Multidisciplinary) 2O
2O /electrolyte interface during lithium battery reaction
/electrolyte interface during lithium battery reactionHirayama, Masaaki*; Ido, Hidekazu*; Kim, K.-S.*; Cho, W.*; Tamura, Kazuhisa; Mizuki, Junichiro; Kanno, Ryoji*
Journal of the American Chemical Society, 132(43), p.15268 - 15276, 2010/11
Times Cited Count:335 Percentile:98.03(Chemistry, Multidisciplinary)Epitaxial LiMn O
O thin films with restricted lattice planes (111) and (110) are grown on SrTiO
thin films with restricted lattice planes (111) and (110) are grown on SrTiO substrates by pulsed laser deposition. In situ SXRD studies have revealed dynamic structural changes that reduce the atomic symmetry at the electrode surface during the initial electrochemical reaction. The surface structural changes commence with the formation of an electric double layer, which is followed by surface reconstruction when a voltage is applied in the first charge process. Transmission electron microscopy images after 10 cycles confirm the formation of a solid electrolyte interface (SEI) layer on both the (111) and (110) surfaces and Mn dissolution from the (110) surface. The (111) surface is more stable than the (110) surface. The electrode stability of LiMn
substrates by pulsed laser deposition. In situ SXRD studies have revealed dynamic structural changes that reduce the atomic symmetry at the electrode surface during the initial electrochemical reaction. The surface structural changes commence with the formation of an electric double layer, which is followed by surface reconstruction when a voltage is applied in the first charge process. Transmission electron microscopy images after 10 cycles confirm the formation of a solid electrolyte interface (SEI) layer on both the (111) and (110) surfaces and Mn dissolution from the (110) surface. The (111) surface is more stable than the (110) surface. The electrode stability of LiMn O
O depends on the reaction rate of SEI formation and the stability of the reconstructed surface structure.
depends on the reaction rate of SEI formation and the stability of the reconstructed surface structure.
Ribas-Arino, J.*; Shiga, Motoyuki; Marx, D.*
Journal of the American Chemical Society, 132(30), p.10609 - 10614, 2010/08
Times Cited Count:89 Percentile:85.63(Chemistry, Multidisciplinary)We present a theoretical study on the role played by aliphatic polymer chains in the transduction of external forces to mechanophores. Upon introducing a rigorous approach rooted in catastrophe theory we demonstrate that the rupture force of a cis 1,2 disubstituted benzocyclobutene features a remarkable dependence on the length of attached polymer chains. Not only do our findings highlight the necessity of taking into account the polymer chains explicitly when thinking about mechanochemical manipulation, but they also announce the possibility of tuning the properties of mechanoresponsive polymers by tailoring the force-transducing chain molecules.
Uchida, Yoshiaki*; Suzuki, Katsuaki*; Tamura, Rui*; Ikuma, Naohiko*; Shimono, Satoshi*; Noda, Yohei; Yamauchi, Jun*
Journal of the American Chemical Society, 132(28), p.9746 - 9752, 2010/06
Times Cited Count:53 Percentile:75.31(Chemistry, Multidisciplinary)An anisotropic and inhomogeneous magnetic interaction (the average spin-spin interaction constant, 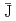
 0) was observed in the various liquid crystalline (LC) phases of racemic and nonracemic all-organic radical LC compounds. We discussed how the LC superstructures induced the magnetic interaction to operate in the LC phases in terms of spin-spin dipole and exchange interactions by means of VT-EPR spectroscopy. The magnitude of the magnetic interaction depended on the type of LC phase, or the superstructure. Furthermore, these radical LC droplets floating on water were commonly attracted to a permanent magnet and moved freely under the influence of this magnet, whereas the crystallized particles of the same compounds never responded to the magnet. The response of the LC droplets to the magnet also varied depending on the type of LC phase, that is, the extent of the magnetic interaction.
0) was observed in the various liquid crystalline (LC) phases of racemic and nonracemic all-organic radical LC compounds. We discussed how the LC superstructures induced the magnetic interaction to operate in the LC phases in terms of spin-spin dipole and exchange interactions by means of VT-EPR spectroscopy. The magnitude of the magnetic interaction depended on the type of LC phase, or the superstructure. Furthermore, these radical LC droplets floating on water were commonly attracted to a permanent magnet and moved freely under the influence of this magnet, whereas the crystallized particles of the same compounds never responded to the magnet. The response of the LC droplets to the magnet also varied depending on the type of LC phase, that is, the extent of the magnetic interaction.
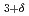
Belik, A. A.*; Kodama, Katsuaki; Igawa, Naoki; Shamoto, Shinichi; Kosuda, Kosuke*; Muromachi, Eiji*
Journal of the American Chemical Society, 132(23), p.8137 - 8144, 2010/05
Times Cited Count:50 Percentile:73.99(Chemistry, Multidisciplinary)Long, Y.*; Saito, Takashi*; Mizumaki, Masaichiro*; Agui, Akane; Shimakawa, Yuichi*
Journal of the American Chemical Society, 131(44), p.16244 - 16247, 2009/10
Times Cited Count:64 Percentile:80.57(Chemistry, Multidisciplinary)A-site-ordered perovskites LaMn Cr
Cr O
O and LaMn
and LaMn Ti
Ti O
O were synthesized under high-pressure and high-temperature conditions. The charge formula of LaMn
were synthesized under high-pressure and high-temperature conditions. The charge formula of LaMn Cr
Cr O
O was found to be LaMn
was found to be LaMn Cr
Cr O
O with Mn
with Mn at the square-coordinated A site. The valence of the A-site Mn ions in LaMnTi
at the square-coordinated A site. The valence of the A-site Mn ions in LaMnTi O
O appeared to be less than +2, and the charge combination in this compound seemed to be LaMnTi
appeared to be less than +2, and the charge combination in this compound seemed to be LaMnTi O
O . Although the square-coordinated A site in A-site-ordered perovskites has been widely known to be occupied by Jahn-Teller active Mn
. Although the square-coordinated A site in A-site-ordered perovskites has been widely known to be occupied by Jahn-Teller active Mn , the present results show that the valence of Mn at the A site can vary from +3 to possible +1.67.
, the present results show that the valence of Mn at the A site can vary from +3 to possible +1.67.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka*; Tsukada, Kazuaki; Asai, Masato; Kitatsuji, Yoshihiro; Ishii, Yasuo; Tome, Hayato; Nishinaka, Ichiro; Haba, Hiromitsu*; Oe, Kazuhiro*; et al.
Journal of the American Chemical Society, 131(26), p.9180 - 9181, 2009/07
Times Cited Count:16 Percentile:49.43(Chemistry, Multidisciplinary)We report here on the successful oxidation of element 102, nobelium (No), on an atom-at-a-time scale in 0.1 M  -hydroxyisobutyric acid (
-hydroxyisobutyric acid ( -HIB) solution using newly developed flow electrolytic column chromatography. It is found that the most stable No
-HIB) solution using newly developed flow electrolytic column chromatography. It is found that the most stable No is oxidized to No
is oxidized to No within 3 min and that the oxidized No complex with
within 3 min and that the oxidized No complex with  -HIB holds the trivalent state in the column above the applied potential of 1.0 V.
-HIB holds the trivalent state in the column above the applied potential of 1.0 V.
Tamada, Taro; Kinoshita, Takayoshi*; Kurihara, Kazuo; Adachi, Motoyasu; Ohara, Takashi; Imai, Keisuke*; Kuroki, Ryota; Tada, Toshiji*
Journal of the American Chemical Society, 131(31), p.11033 - 11040, 2009/07
Times Cited Count:61 Percentile:79.62(Chemistry, Multidisciplinary)To help resolve long-standing questions regarding the catalytic activity of the serine proteases the structure of porcine pancreatic elastase has been analyzed by high-resolution neutron and X-ray crystallography. In order to mimic the tetrahedral transition intermediate a peptidic inhibitor was used. A single large crystal was used to collect room-temperature neutron data to 1.65  resolution and X-ray data to 1.20
resolution and X-ray data to 1.20  resolution. Another crystal provided a low-temperature X-ray data set to 0.94
resolution. Another crystal provided a low-temperature X-ray data set to 0.94  resolution. The neutron data are to higher resolution than previously reported for a serine protease and the X-ray data are comparable with other studies. The neutron and X-ray data show that the hydrogen bond between His57 and Asp102 (chymotrypsin numbering) is 2.60
resolution. The neutron data are to higher resolution than previously reported for a serine protease and the X-ray data are comparable with other studies. The neutron and X-ray data show that the hydrogen bond between His57 and Asp102 (chymotrypsin numbering) is 2.60  in length and that the hydrogen-bonding hydrogen is 0.80-0.96
in length and that the hydrogen-bonding hydrogen is 0.80-0.96  from the histidine nitrogen. This is not consistent with a low-barrier hydrogen which is predicted to have the hydrogen midway between the donor and acceptor atom. The observed interaction between His57 and Asp102 is essentially a short but conventional hydrogen bond, sometimes described as a short ionic hydrogen bond. The neutron analysis also shows that the oxygen of the oxopropyl group of the inhibitor is present as an oxygen anion rather than a hydroxyl group, supporting the role of the "oxyanion hole" in stabilizing the tetrahedral intermediate in catalysis.
from the histidine nitrogen. This is not consistent with a low-barrier hydrogen which is predicted to have the hydrogen midway between the donor and acceptor atom. The observed interaction between His57 and Asp102 is essentially a short but conventional hydrogen bond, sometimes described as a short ionic hydrogen bond. The neutron analysis also shows that the oxygen of the oxopropyl group of the inhibitor is present as an oxygen anion rather than a hydroxyl group, supporting the role of the "oxyanion hole" in stabilizing the tetrahedral intermediate in catalysis.