Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 in LiCl-Li
in LiCl-Li O melt
O meltSakamura, Yoshiharu*; Murakami, Tsuyoshi*; Iizuka, Masatoshi*; Kofuji, Hirohide
Journal of the Electrochemical Society, 169(6), p.063504_1 - 063504_13, 2022/06
Times Cited Count:2 Percentile:9.97(Electrochemistry)The development of an O -evolving inert anode is of crucial importance for the electrolytic reduction process of oxide nuclear fuels using LiCl-Li
-evolving inert anode is of crucial importance for the electrolytic reduction process of oxide nuclear fuels using LiCl-Li O melts at 923 K. As scaled-up anodes for practical use, metallic anodes are preferable. In this study, Fe, Ni, and Fe-Ni metals were electrochemically examined and the results indicate that Ni metal coated with NiO is a promising anode material.
O melts at 923 K. As scaled-up anodes for practical use, metallic anodes are preferable. In this study, Fe, Ni, and Fe-Ni metals were electrochemically examined and the results indicate that Ni metal coated with NiO is a promising anode material.
Matsumiya, Masahiko*; Tsuchida, Yusuke*; Kinoshita, Ryoma*; Sasaki, Yuji
Journal of the Electrochemical Society, 168(7), p.076508_1 - 076508_6, 2021/07
Times Cited Count:0 Percentile:0.00(Electrochemistry)It is important to develop solvent extraction and electrodeposition processes for platinum group metals in order to reduce the volume of secondary wastes. In this study, the electrodeposition behavior of the extracted Pt(IV) complex in a phosphonium-based ionic liquid (IL) was investigated by using an electrochemical quartz crystal microbalance (EQCM). The charge transfer reaction Pt(IV) + 2e(-)  Pt(II) was observed at -0.53 V and the electrodeposition reaction Pt(II) + 2e(-)
Pt(II) was observed at -0.53 V and the electrodeposition reaction Pt(II) + 2e(-)  Pt(0) proceeded at -1.65 V in this system. Moreover, consecutive solvent extraction and electrodeposition of Pt metal in Alamine336/IL system were performed at 10 cycles. High extraction percentage (E
Pt(0) proceeded at -1.65 V in this system. Moreover, consecutive solvent extraction and electrodeposition of Pt metal in Alamine336/IL system were performed at 10 cycles. High extraction percentage (E  95.1 %) and good current efficiency (
95.1 %) and good current efficiency (
 85.8 %) were maintained in the first to sixth cycles. The XPS Pt-4f (7/2) spectrum confirmed that all the electrodeposits were in the metallic state.
85.8 %) were maintained in the first to sixth cycles. The XPS Pt-4f (7/2) spectrum confirmed that all the electrodeposits were in the metallic state.
Matsumiya, Masahiko*; Nomizu, Daiki*; Tsuchida, Yusuke*; Sasaki, Yuji
Journal of the Electrochemical Society, 168(5), p.056502_1 - 056502_7, 2021/05
Times Cited Count:1 Percentile:2.37(Electrochemistry)The coordination states of multivalent dysprosium complexes of potassium bis(trifluoromethylsulfonyl) amide (K[NTf ]) were investigated by Raman spectroscopy. The solvation number, n, of the dysprosium complexes was determined to be 4.12 for Dy(II) and 5.09 for Dy(III). Electrochemical analysis revealed that the reduction peak of [Dy-III(NTf
]) were investigated by Raman spectroscopy. The solvation number, n, of the dysprosium complexes was determined to be 4.12 for Dy(II) and 5.09 for Dy(III). Electrochemical analysis revealed that the reduction peak of [Dy-III(NTf )(5)](2-) at approximately +0.81 V at 483 K is based on an electrodeposition reaction from [Dy-III(NTf
)(5)](2-) at approximately +0.81 V at 483 K is based on an electrodeposition reaction from [Dy-III(NTf )(5)](2-) to Dy(0). The nucleation behavior of [Dy-III(NTf
)(5)](2-) to Dy(0). The nucleation behavior of [Dy-III(NTf )(5)](2-) was evaluated using chronoamperometry. The results indicated that the nucleation mechanism of Dy nuclei changed from instantaneous to progressive nucleation when the overpotential became more negative than the deposition potential of Dy(0). The electrodeposits were identified as mostly the metallic state by X-ray photoelectron spectroscopy.
)(5)](2-) was evaluated using chronoamperometry. The results indicated that the nucleation mechanism of Dy nuclei changed from instantaneous to progressive nucleation when the overpotential became more negative than the deposition potential of Dy(0). The electrodeposits were identified as mostly the metallic state by X-ray photoelectron spectroscopy.
Matsumiya, Masahiko*; Kinoshita, Ryoma*; Tsuchida, Yusuke*; Sasaki, Yuji
Journal of the Electrochemical Society, 168(5), p.056501_1 - 056501_6, 2021/05
Times Cited Count:9 Percentile:38.88(Electrochemistry)The extraction behavior of Ir(IV) in a phosphonium-based ionic liquid (IL) system, triethyl-n-pentyl phosphonium bis(trifluoromethyl-sulfonyl)amide ([P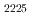 ][NTf
][NTf ]), was investigated using an amine extractant. Voltammetry-based electrochemical analysis revealed that the reduction of Ir(IV) proceeded via an intermediate Ir(III) species in two steps, namely, Ir(IV) + e(-)
]), was investigated using an amine extractant. Voltammetry-based electrochemical analysis revealed that the reduction of Ir(IV) proceeded via an intermediate Ir(III) species in two steps, namely, Ir(IV) + e(-)  Ir(III) and Ir(III) + 3e(-)
Ir(III) and Ir(III) + 3e(-)  Ir(0). Diffusion coefficients of the extracted Ir(IV) species in the IL were determined over the temperature range of 298-373 K using semi-integral and semi-differential analyses, and values obtained from the two analyses were consistent with each other. Furthermore, consecutive solvent extraction and electrodeposition of Ir metal in the IL bath were performed for 10 cycles. The entire Ir electrodeposit was in the metallic state, as is evident from the 4f (7/2) spectrum obtained by XPS.
Ir(0). Diffusion coefficients of the extracted Ir(IV) species in the IL were determined over the temperature range of 298-373 K using semi-integral and semi-differential analyses, and values obtained from the two analyses were consistent with each other. Furthermore, consecutive solvent extraction and electrodeposition of Ir metal in the IL bath were performed for 10 cycles. The entire Ir electrodeposit was in the metallic state, as is evident from the 4f (7/2) spectrum obtained by XPS.

 generation; The Role of NO
generation; The Role of NO
 in the crevice corrosion repassivation of type 316L stainless steel
in the crevice corrosion repassivation of type 316L stainless steelAoyama, Takahito; Sugawara, Yu*; Muto, Izumi*; Hara, Nobuyoshi*
Journal of the Electrochemical Society, 166(10), p.C250 - C260, 2019/01
Times Cited Count:5 Percentile:13.00(Electrochemistry)The role of NO
 in the repassivation of crevice corrosion of Type 316L stainless steel was investigated. In crevice corrosion tests, the solution was changed from 1 M NaCl to NaCl-NaNO
in the repassivation of crevice corrosion of Type 316L stainless steel was investigated. In crevice corrosion tests, the solution was changed from 1 M NaCl to NaCl-NaNO . NO
. NO
 led to complete repassivation. Repassivation of the crevice corrosion was found to take place in two steps. In the first step, the estimated current density inside the crevice gradually decreased from ca. 5 mA cm
led to complete repassivation. Repassivation of the crevice corrosion was found to take place in two steps. In the first step, the estimated current density inside the crevice gradually decreased from ca. 5 mA cm to ca. 5
to ca. 5  A cm
A cm . After that, the current density suddenly decreased to less than 0.1
. After that, the current density suddenly decreased to less than 0.1  A cm
A cm . From the potentiodynamic polarization in acidic solutions simulated inside the crevice (pH 0.2) and in situ observations of the crevice corrosion morphology, the first step was thought to be generated by the suppression of active dissolution by NO
. From the potentiodynamic polarization in acidic solutions simulated inside the crevice (pH 0.2) and in situ observations of the crevice corrosion morphology, the first step was thought to be generated by the suppression of active dissolution by NO
 . It would appear that the generation of NH
. It would appear that the generation of NH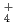 results in a pH increase and the further suppression of active dissolution, and then repassivation occurs.
results in a pH increase and the further suppression of active dissolution, and then repassivation occurs.
 O
O /C hydrazine electrooxidation catalysts for anion exchange membrane fuel cells
/C hydrazine electrooxidation catalysts for anion exchange membrane fuel cellsSakamoto, Tomokazu*; Masuda, Teruyuki*; Yoshimoto, Koji*; Kishi, Hirofumi*; Yamaguchi, Susumu*; Matsumura, Daiju; Tamura, Kazuhisa; Hori, Akihiro*; Horiuchi, Yosuke*; Serov, A.*; et al.
Journal of the Electrochemical Society, 164(4), p.F229 - F234, 2017/01
Times Cited Count:13 Percentile:38.72(Electrochemistry)Sakamoto, Tomokazu*; Kishi, Hirofumi*; Yamaguchi, Susumu*; Matsumura, Daiju; Tamura, Kazuhisa; Hori, Akihiro*; Horiuchi, Yosuke*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; et al.
Journal of the Electrochemical Society, 163(10), p.H951 - H957, 2016/08
Times Cited Count:33 Percentile:75.66(Electrochemistry) O
O epitaxial thin film electrode for lithium batteries
epitaxial thin film electrode for lithium batteriesSuzuki, Kota*; Hirayama, Masaaki*; Kim, K.-S.*; Taminato, So*; Tamura, Kazuhisa; Son, J.-Y.*; Mizuki, Junichiro; Kanno, Ryoji*
Journal of the Electrochemical Society, 162(13), p.A7083 - A7090, 2015/08
Times Cited Count:12 Percentile:36.26(Electrochemistry)The effects of surface coatings on LiMn O
O were investigated using LiMn
were investigated using LiMn O
O epitaxial thin films with a thickness of 30 nm. Bare and surface-coated LiMn
epitaxial thin films with a thickness of 30 nm. Bare and surface-coated LiMn O
O epitaxial thin films were synthesized on SrTiO
epitaxial thin films were synthesized on SrTiO (111) substrates using a pulsed laser deposition method. The surface coating, which was formed using the solid electrolyte Li
(111) substrates using a pulsed laser deposition method. The surface coating, which was formed using the solid electrolyte Li PO
PO and had a thickness of 3 nm, improved the reversibility of the electrochemical reactions undergone by the LiMn
and had a thickness of 3 nm, improved the reversibility of the electrochemical reactions undergone by the LiMn O
O epitaxial thin films. The changes induced in the surface structure were maintained during battery operation; in contrast, the bare LiMn
epitaxial thin films. The changes induced in the surface structure were maintained during battery operation; in contrast, the bare LiMn O
O thin film exhibited structural degradation and Mn dissolution. The structural changes induced in the coated electrode and the increase in its surface stability were intrinsic effects of the Li
thin film exhibited structural degradation and Mn dissolution. The structural changes induced in the coated electrode and the increase in its surface stability were intrinsic effects of the Li PO
PO coating and improved the electrochemical performance of the LiMn
coating and improved the electrochemical performance of the LiMn O
O thin-film electrode.
thin-film electrode.
Yoshimura, Kimio; Koshikawa, Hiroshi; Yamaki, Tetsuya; Shishitani, Hideyuki*; Yamamoto, Kazuya*; Yamaguchi, Susumu*; Tanaka, Hirohisa*; Maekawa, Yasunari
Journal of the Electrochemical Society, 161(9), p.F889 - F893, 2014/06
Times Cited Count:23 Percentile:61.70(Electrochemistry)Graft-type anion-conducting electrolyte membranes (AEMs) with imidazolium cations on graft polymers were synthesized through radiation-induced graft polymerization of  -vinylimidazole (NVIm) on poly(ethylene-co-tetrafluoroethylene) (ETFE) films, followed by
-vinylimidazole (NVIm) on poly(ethylene-co-tetrafluoroethylene) (ETFE) films, followed by  -propylation and ion-exchange reactions. The
-propylation and ion-exchange reactions. The  -propylation proceeded quantitatively, whereas the ion-exchange reactions in 1 M KOH at 60
-propylation proceeded quantitatively, whereas the ion-exchange reactions in 1 M KOH at 60 C were accompanied by partial
C were accompanied by partial  -elimination of the imidazolium cations(AEM2), which exhibited an ion-exchange capacity (IEC) of 0.85 mmol g
-elimination of the imidazolium cations(AEM2), which exhibited an ion-exchange capacity (IEC) of 0.85 mmol g and ionic conductivity of 10 mS cm
and ionic conductivity of 10 mS cm . AEM2 showed alkaline stability at 60
. AEM2 showed alkaline stability at 60 C but it gradually degraded at 80
C but it gradually degraded at 80 C for ca. 150 h. The copolymer-type AEM (AEM3) with an IEC of 1.20 mmol g
C for ca. 150 h. The copolymer-type AEM (AEM3) with an IEC of 1.20 mmol g was prepared through the copolymerization of NVIm with styrene on ETFE films, followed by the same
was prepared through the copolymerization of NVIm with styrene on ETFE films, followed by the same  -propylation and ion-exchange reactions. AEM3 was shown higher alkaline durability in 1 M KOH at 80
-propylation and ion-exchange reactions. AEM3 was shown higher alkaline durability in 1 M KOH at 80 C. As a result, it exhibited higher conductivity (
C. As a result, it exhibited higher conductivity ( 10 mS cm
10 mS cm ) for 250 h. Therefore, alkylimidazolium cations in copolymer grafts are a promising anion conducting group for alkaline-durable AEMs. A maximum power density of 75 mW cm
) for 250 h. Therefore, alkylimidazolium cations in copolymer grafts are a promising anion conducting group for alkaline-durable AEMs. A maximum power density of 75 mW cm is obtained for AEM3 in a direct hydrazine hydrate fuel cell.
is obtained for AEM3 in a direct hydrazine hydrate fuel cell.
Soma, Yasutaka; Kato, Chiaki; Yamamoto, Masahiro
Journal of the Electrochemical Society, 159(8), p.C334 - C340, 2012/07
Times Cited Count:10 Percentile:33.21(Electrochemistry)Surface oxide layers on stainless steel were formed in 561 K pure water at different potentials. To understand the oxide's properties in terms of their potential dependence, cross-sectional views of the oxide layer were analyzed using an electron microprobe technique and potential-solubility (equilibrium concentration of ionic species) diagram. In the potential range investigated, duplex oxide layers composed of mono- and bimetallic oxide were formed. Both the structure and composition of the oxide layer were affected by solubility of oxides.
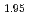 Mn
Mn Co
Co O
O (
(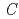 2/
2/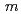 ) as a lithium-battery cathode
) as a lithium-battery cathodeOzawa, Kiyoshi*; Nakao, Yasuhiro*; Mochiku, Takashi*; Cheng, Z.*; Wang, L.*; Iwai, Hideo*; Tsuchiya, Yoshinori*; Fujii, Hiroki*; Igawa, Naoki
Journal of the Electrochemical Society, 159(3), p.A300 - A304, 2012/01
Times Cited Count:14 Percentile:44.59(Electrochemistry)A manganese-based solid solution with the composition of Li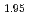 Mn
Mn Co
Co O
O was synthesized by a simplified coprecipitation method, and its electrochemical characteristics as a lithium-battery cathode were investigated. Rietveld refinement based on neutron diffraction data revealed that the material is assigned to an Li
was synthesized by a simplified coprecipitation method, and its electrochemical characteristics as a lithium-battery cathode were investigated. Rietveld refinement based on neutron diffraction data revealed that the material is assigned to an Li MnO
MnO -type structure model with a space group symmetry of
-type structure model with a space group symmetry of 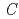 2/
2/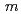 . In cycling of the cell in the potential range from 2.0 to 4.8 V at current densities of 30 mAhg
. In cycling of the cell in the potential range from 2.0 to 4.8 V at current densities of 30 mAhg , the discharge capacity characteristically increases from 46.3 to 196.5 mAhg
, the discharge capacity characteristically increases from 46.3 to 196.5 mAhg as the cycle increases from 1 to 11, and a discharge capacity above 175.5 mAhg
as the cycle increases from 1 to 11, and a discharge capacity above 175.5 mAhg is obtained between the 23rd and 58th cycles. The cyclic voltammogram and X-ray photoelectron spectroscopy measurements showed that the manganese redox reaction is progressively activated during the first ten-odd cycles.
is obtained between the 23rd and 58th cycles. The cyclic voltammogram and X-ray photoelectron spectroscopy measurements showed that the manganese redox reaction is progressively activated during the first ten-odd cycles.
Ben youcef, H.*; Gubler, L.*; Yamaki, Tetsuya; Sawada, Shinichi; Alkan G rsel, S.*; Wokaun, A.*; Scherer, G. G.*
rsel, S.*; Wokaun, A.*; Scherer, G. G.*
Journal of the Electrochemical Society, 156(4), p.B532 - B539, 2009/02
Times Cited Count:17 Percentile:51.41(Electrochemistry)The effect of cross-linker content on the chemical stability of poly(ethylene- -tetrafluoroethylene) (ETFE)-based radiation-grafted and sulfonated membranes was investigated. An ex situ degradation test in hydrogen peroxide solution showed a strong increase in stability of crosslinked membranes compared to uncrosslinked ones. Excessive crosslinking, however, is detrimental to the chemical and mechanical properties. Furthermore, the stability of grafted membranes based on ETFE was superior to those based on poly(tetrafluoroethylene-
-tetrafluoroethylene) (ETFE)-based radiation-grafted and sulfonated membranes was investigated. An ex situ degradation test in hydrogen peroxide solution showed a strong increase in stability of crosslinked membranes compared to uncrosslinked ones. Excessive crosslinking, however, is detrimental to the chemical and mechanical properties. Furthermore, the stability of grafted membranes based on ETFE was superior to those based on poly(tetrafluoroethylene- -hexafluoropropylene) (FEP). An in situ long-term test in a hydrogen/oxygen single cell over 2180 h, using an ETFE-based grafted membrane optimized with respect to cross-linker content, with a graft level of 25% was carried out. The performance of the membrane electrode assembly exhibited a voltage decay rate of 13
-hexafluoropropylene) (FEP). An in situ long-term test in a hydrogen/oxygen single cell over 2180 h, using an ETFE-based grafted membrane optimized with respect to cross-linker content, with a graft level of 25% was carried out. The performance of the membrane electrode assembly exhibited a voltage decay rate of 13  V/h over the testing time at a current density of 500 mA/cm
V/h over the testing time at a current density of 500 mA/cm and a cell temperature of 80
and a cell temperature of 80 C, while the hydrogen permeation showed a steady increase over time. This indicates that, to some extent, changes in the membrane morphology occur over the operating period. Local postmortem analysis of the tested membrane reveals that high degradation was observed in areas adjacent to the oxygen inlet and in other areas nearby.
C, while the hydrogen permeation showed a steady increase over time. This indicates that, to some extent, changes in the membrane morphology occur over the operating period. Local postmortem analysis of the tested membrane reveals that high degradation was observed in areas adjacent to the oxygen inlet and in other areas nearby.
 O
O electrode
electrodeHirayama, Masaaki*; Sonoyama, Noriyuki*; Ito, Masumi*; Minoura, Machiko*; Mori, Daisuke*; Yamada, Atsuo*; Tamura, Kazuhisa; Mizuki, Junichiro; Kanno, Ryoji*
Journal of the Electrochemical Society, 154(11), p.A1065 - A1072, 2007/00
Times Cited Count:101 Percentile:95.13(Electrochemistry)Structural changes at electrode/electrolyte interface of a lithium cell were studied by X-ray reflectometry and two-dimensional model electrodes with a restricted lattice plane of LiMn O
O . The ex situ reflectometry indicated that a thin impurity layer covered the lattice plane of the as-grown film. The impurity layer was dissolved and a solid-electrolyte-interface-like phase appeared after the electrode was soaked into the electrolyte. The in situ observation clarified that the surface reactivity depended on the lattice planes of the spinel; the defect layer at the (111) plane was stable during the electrochemical reaction, whereas a slight decrease in the film thickness was observed for the (110) plane. Our surface characterization of the intercalation electrode indicated that the surface structure changes during the pristine stage of the change-discharge processes and these changes are dependent on the lattice orientation of LiMn
. The ex situ reflectometry indicated that a thin impurity layer covered the lattice plane of the as-grown film. The impurity layer was dissolved and a solid-electrolyte-interface-like phase appeared after the electrode was soaked into the electrolyte. The in situ observation clarified that the surface reactivity depended on the lattice planes of the spinel; the defect layer at the (111) plane was stable during the electrochemical reaction, whereas a slight decrease in the film thickness was observed for the (110) plane. Our surface characterization of the intercalation electrode indicated that the surface structure changes during the pristine stage of the change-discharge processes and these changes are dependent on the lattice orientation of LiMn O
O .
.
 and UCl
and UCl dissolved in a LiCl-KCl eutectic melt
dissolved in a LiCl-KCl eutectic meltKuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Journal of the Electrochemical Society, 152(4), p.C203 - C212, 2005/04
Times Cited Count:117 Percentile:95.02(Electrochemistry)The electrochemical behavior of UCl and UCl
and UCl dissolved in LiCl-KCl eutectic melt was studied at 723-823 K. Electroreduction of U(IV) in LiCl-KCl melt occurs via two successive steps involving transfer of one and three electrons. The diffusion coefficients of U(IV) and U(III) ions were determined by linear sweep voltammetry, chronopotentiometry, and chronoamperometry. The formal standard potential of E
dissolved in LiCl-KCl eutectic melt was studied at 723-823 K. Electroreduction of U(IV) in LiCl-KCl melt occurs via two successive steps involving transfer of one and three electrons. The diffusion coefficients of U(IV) and U(III) ions were determined by linear sweep voltammetry, chronopotentiometry, and chronoamperometry. The formal standard potential of E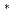 U(IV)/U(III), E
U(IV)/U(III), E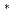 U(IV)/U, and E
U(IV)/U, and E U(III)/U were determined and some thermodynamic properties of UCl
U(III)/U were determined and some thermodynamic properties of UCl and UCl
and UCl dissolved in LiCl-KCl eutectic melt were calculated. Influence of oxide ions on electrochemical behavior was also studied.
dissolved in LiCl-KCl eutectic melt were calculated. Influence of oxide ions on electrochemical behavior was also studied.
Kawamura, Hiroyuki; Takahashi, Masamitsu; Mizuki, Junichiro
Journal of the Electrochemical Society, 149(11), p.C586 - C591, 2002/11
Times Cited Count:14 Percentile:43.57(Electrochemistry)no abstracts in English
Kawamura, Hiroyuki; Takahashi, Masamitsu; Hojo, Nobuhiko*; Miyake, Masao*; Murase, Kuniaki*; Tamura, Kazuhisa*; Uosaki, Kohei*; Awakura, Yasuhiro*; Mizuki, Junichiro; Matsubara, Eiichiro*
Journal of the Electrochemical Society, 149(2), p.C83 - C88, 2002/02
Times Cited Count:6 Percentile:21.37(Electrochemistry)The structure of a Te layer formed on a Au(111) substrate by underpotential deposition (UPD) in an electrolytic solution has been studied using in-situ surface X-ray diffraction technique. The measurements were carried out for a series of samples which were kept at UPD potential for 4 to 59 hours. The results revealed that the Te UPD layer is unstable. The top layer is analyzed to consist of the UPD Te atoms and Au atoms which diffuse from the Au(111) substrate. Also, the Te UPD layer does not have the structure with periodicity reported in previous works, such as (

 ) R30
) R30 after ample time elapses. Stripping voltammetry for the Te UPD layer shows that the interaction between Te and Au increases with time, supporting the finding that the top layer is a mixture of Te and Au.
after ample time elapses. Stripping voltammetry for the Te UPD layer shows that the interaction between Te and Au increases with time, supporting the finding that the top layer is a mixture of Te and Au.
Sakamura, Y.*; Shirai, Osamu; Iwai, Takashi; Suzuki, Yasufumi
Journal of the Electrochemical Society, 147(2), p.642 - 649, 2000/02
Times Cited Count:21 Percentile:61.71(Electrochemistry)no abstracts in English
Monguchi, Takuo*; Fujioka, Hiroshi*; Uragami, Takeshi*; Ono, Kanta*; Baba, Yuji; Oshima, Masaharu*
Journal of the Electrochemical Society, 147(2), p.741 - 743, 2000/02
Times Cited Count:5 Percentile:23.06(Electrochemistry)no abstracts in English
; Kaetsu, Isao;
Journal of the Electrochemical Society, 131(6), p.1272 - 1274, 1984/00
Times Cited Count:4 Percentile:27.18(Electrochemistry)no abstracts in English