Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Bateman, K.*; Murayama, Shota*; Hanamachi, Yuji*; Wilson, J.*; Seta, Takamasa*; Amano, Yuki; Kubota, Mitsuru*; Ouchi, Yuji*; Tachi, Yukio
Minerals (Internet), 12(7), p.883_1 - 883_20, 2022/07
Times Cited Count:2 Percentile:20.03(Geochemistry & Geophysics) and Mn
and Mn by fungal manganese oxide through asbolane formation
by fungal manganese oxide through asbolane formationAoshima, Miku*; Tani, Yukinori*; Fujita, Rina*; Tanaka, Kazuya; Miyata, Naoyuki*; Umezawa, Kazuhiro*
Minerals (Internet), 12(3), p.358_1 - 358_16, 2022/03
Times Cited Count:9 Percentile:72.26(Geochemistry & Geophysics)In this study, we conducted sequestration experiments of Co by
by 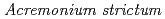 KR21-2 in a Mn
KR21-2 in a Mn /Co
/Co binary solution at pH7. The sequestration of Co
binary solution at pH7. The sequestration of Co by newly formed BMOs readily progressed in parallel with the exogenous Mn
by newly formed BMOs readily progressed in parallel with the exogenous Mn , with higher efficiency than that in single Co
, with higher efficiency than that in single Co solutions. This is attributed to a synergetic effect on Co
solutions. This is attributed to a synergetic effect on Co sequestration through the formation of asbolane in Mn
sequestration through the formation of asbolane in Mn /Co
/Co binary systems.
binary systems.
Sawada, Hikaru*; Niki, Sota*; Nagata, Mitsuhiro; Hirata, Takafumi*
Minerals (Internet), 12(1), p.107_1 - 107_15, 2022/01
Times Cited Count:5 Percentile:49.13(Geochemistry & Geophysics)The Oeyama ophiolite is one of the oldest components in Japanese Islands and important to reveal the initiation of plate subduction along proto-Japan. This study performed U-Pb-Hf isotopic and trace element analyses of zircon in gabbroic rocks. The weight mean of  Pb/
Pb/ U dates from zircons of the Oeyama ophiolite is 544
U dates from zircons of the Oeyama ophiolite is 544  4 Ma (2 sigma). Trace element analysis of the zircons exhibit that the host rock was derived from the mantle depleted of incompatible elements like mid-oceanic ridge basalt. The present igneous age and geochemical feature of the zircons is consistent with previous work for other part of the Oeyama ophiolite. Zircon Lu-Hf isotopic analysis also indicate that the gabbroic rock was derived from the depleted mantle domain. The Hf isotopic signature is more depleted than those of the zircons in jadeitite associated with the Oeyama ophiolite. This result implies that the older crustal material was involved into the initial oceanic plate subduction along the proto-Japan arc.
4 Ma (2 sigma). Trace element analysis of the zircons exhibit that the host rock was derived from the mantle depleted of incompatible elements like mid-oceanic ridge basalt. The present igneous age and geochemical feature of the zircons is consistent with previous work for other part of the Oeyama ophiolite. Zircon Lu-Hf isotopic analysis also indicate that the gabbroic rock was derived from the depleted mantle domain. The Hf isotopic signature is more depleted than those of the zircons in jadeitite associated with the Oeyama ophiolite. This result implies that the older crustal material was involved into the initial oceanic plate subduction along the proto-Japan arc.
 U accumulations in
U accumulations in 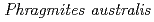 naturally growing at the mill tailings pond; Iron plaque formation possibly related to root-endophytic bacteria producing siderophores
naturally growing at the mill tailings pond; Iron plaque formation possibly related to root-endophytic bacteria producing siderophoresNakamoto, Yukihiro*; Doyama, Kohei*; Haruma, Toshikatsu*; Lu, X.*; Tanaka, Kazuya; Kozai, Naofumi; Fukuyama, Kenjin; Fukushima, Shigeru; Ohara, Yoshiyuki; Yamaji, Keiko*
Minerals (Internet), 11(12), p.1337_1 - 1337_17, 2021/12
Times Cited Count:5 Percentile:35.78(Geochemistry & Geophysics)Mine drainage is a vital water problem in the mining industry worldwide because of the heavy metal elements and low pH. Rhizofiltration using wetland plants is an appropriate method to remove heavy metals from the water via accumulation in the rhizosphere. 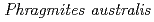 is one of the candidate plants for this method because of metal accumulation, forming iron plaque around the roots. At the study site, which was the mill tailings pond in the Ningyo-toge uranium mine,
is one of the candidate plants for this method because of metal accumulation, forming iron plaque around the roots. At the study site, which was the mill tailings pond in the Ningyo-toge uranium mine, 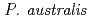 has been naturally growing since 1998. The results showed that
has been naturally growing since 1998. The results showed that 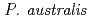 accumulated Fe, Mn, and
accumulated Fe, Mn, and  U in the nodal roots without/with iron plaque compared with other plant tissues. Among the 837 bacterial colonies isolated from nodal roots, 88.6% showed siderophore production activities. Considering iron plaque formation around
U in the nodal roots without/with iron plaque compared with other plant tissues. Among the 837 bacterial colonies isolated from nodal roots, 88.6% showed siderophore production activities. Considering iron plaque formation around 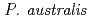 roots, we hypothesized that microbial siderophores might influence iron plaque formation because bacterial siderophores have catechol-like functional groups. The complex of catechol or other phenolics with Fe was precipitated due to the networks between Fe and phenolic derivatives. The experiment using bacterial products of root endophytes, such as
roots, we hypothesized that microbial siderophores might influence iron plaque formation because bacterial siderophores have catechol-like functional groups. The complex of catechol or other phenolics with Fe was precipitated due to the networks between Fe and phenolic derivatives. The experiment using bacterial products of root endophytes, such as 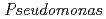 spp. and
spp. and 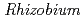 spp., showed precipitation with Fe ions, and we confirmed that several
spp., showed precipitation with Fe ions, and we confirmed that several 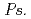 spp. and
spp. and 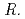 spp. produced unidentified phenolic compounds. In conclusion, root-endophytic bacteria such as
spp. produced unidentified phenolic compounds. In conclusion, root-endophytic bacteria such as 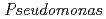 spp. and
spp. and 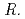 spp., isolated from metal-accumulating roots of
spp., isolated from metal-accumulating roots of 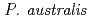 , might influence iron plaque formation as the metal accumulation site. Iron plaque formation is related to tolerance in
, might influence iron plaque formation as the metal accumulation site. Iron plaque formation is related to tolerance in 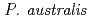 , and
, and 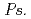 spp. and
spp. and 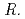 spp. might indirectly contribute to tolerance.
spp. might indirectly contribute to tolerance.
M ri, A.*; Mazurek, M.*; Ota, Kunio; Siitari-Kauppi, M.*; Eichinger, F.*; Leuenberger, M.*
ri, A.*; Mazurek, M.*; Ota, Kunio; Siitari-Kauppi, M.*; Eichinger, F.*; Leuenberger, M.*
Minerals (Internet), 11(10), p.1072_1 - 1072_17, 2021/10
Times Cited Count:5 Percentile:28.73(Geochemistry & Geophysics)Bateman, K.; Murayama, Shota*; Hanamachi, Yuji*; Wilson, J.*; Seta, Takamasa*; Amano, Yuki; Kubota, Mitsuru*; Ouchi, Yuji*; Tachi, Yukio
Minerals (Internet), 11(9), p.1026_1 - 1026_23, 2021/09
Times Cited Count:2 Percentile:6.20(Geochemistry & Geophysics)Bateman, K.; Amano, Yuki; Kubota, Mitsuru*; Ouchi, Yuji*; Tachi, Yukio
Minerals (Internet), 11(6), p.588_1 - 588_19, 2021/06
Times Cited Count:4 Percentile:20.78(Geochemistry & Geophysics) through irreversible biogenic manganese oxide sequestration
through irreversible biogenic manganese oxide sequestrationTani, Yukinori*; Kakinuma, Satomi*; Chang, J.*; Tanaka, Kazuya; Miyata, Naoyuki*
Minerals (Internet), 11(1), p.53_1 - 53_14, 2021/01
Times Cited Count:5 Percentile:28.73(Geochemistry & Geophysics)In this study, we examined the Ba sequestration potential of enzymatically active biogenic Mn oxides (BMOs) with and without exogenous Mn
sequestration potential of enzymatically active biogenic Mn oxides (BMOs) with and without exogenous Mn . The BMOs readily oxidized exogenous Mn
. The BMOs readily oxidized exogenous Mn to produce another BMO phase, and subsequently sequestered Ba
to produce another BMO phase, and subsequently sequestered Ba at a pH of 7.0, with irreversible Ba
at a pH of 7.0, with irreversible Ba sequestration as the dominant pathway. Extended X-ray absorption fine structure spectroscopy and X-ray diffraction analyses demonstrated alteration from turbostratic to tightly stacked birnessite through possible Ba
sequestration as the dominant pathway. Extended X-ray absorption fine structure spectroscopy and X-ray diffraction analyses demonstrated alteration from turbostratic to tightly stacked birnessite through possible Ba incorporation into the interlayer.
incorporation into the interlayer.
Okeme, I. C.*; Scott, T. B.*; Martin, P. G.*; Satou, Yukihiko; Ojonimi, T. I.*; Olaluwoye, M. O.*
Minerals (Internet), 10(3), p.241_1 - 241_15, 2020/03
Times Cited Count:8 Percentile:46.30(Geochemistry & Geophysics) glass up to 10 GPa
glass up to 10 GPaUrakawa, Satoru*; Inoue, Toru*; Hattori, Takanori; Sano, Asami; Kohara, Shinji*; Wakabayashi, Daisuke*; Sato, Tomoko*; Funamori, Nobumasa*; Funakoshi, Kenichi*
Minerals (Internet), 10(1), p.84_1 - 84_13, 2020/01
Times Cited Count:10 Percentile:55.04(Geochemistry & Geophysics)The structure of hydrous amorphous SiO is fundamental to investigate the effects of water on the physicochemical properties of oxide glasses and magma. The hydrous SiO
is fundamental to investigate the effects of water on the physicochemical properties of oxide glasses and magma. The hydrous SiO glass with 13 wt.% D
glass with 13 wt.% D O was synthesized under high-pressure and high-temperature conditions and its structure was investigated by small angle X-ray scattering, X-ray diffraction, and neutron diffraction experiments at pressures of up to 10 GPa and room temperature. This hydrous glass is separated into a SiO
O was synthesized under high-pressure and high-temperature conditions and its structure was investigated by small angle X-ray scattering, X-ray diffraction, and neutron diffraction experiments at pressures of up to 10 GPa and room temperature. This hydrous glass is separated into a SiO rich major phase and a D
rich major phase and a D O rich minor phase. Medium-range order of the hydrous glass shrinks compared to the anhydrous SiO
O rich minor phase. Medium-range order of the hydrous glass shrinks compared to the anhydrous SiO glass due to disruption of SiO
glass due to disruption of SiO linkage by formation of Si-OD deuterioxyl, while the pressure response is similar. Most of D
linkage by formation of Si-OD deuterioxyl, while the pressure response is similar. Most of D O molecules are in the small domains and hardly penetrate into SiO
O molecules are in the small domains and hardly penetrate into SiO major phase.
major phase.
Takahatake, Yoko; Shibata, Atsuhiro; Nomura, Kazunori; Sato, Tsutomu*
Minerals (Internet), 7(12), p.247_1 - 247_13, 2017/12
Times Cited Count:9 Percentile:41.90(Geochemistry & Geophysics)Hydrous sodium titanate (SrTreat) is able to remove radioactive Sr from Radioactive contaminated water at Fukushima Daiichi Nuclear Power station (F1NPS). Knowing the amount of radioactive nuclides in the used SrTreat is important for an effective disposal and deposition of the F1NPS waste. This study investigated changes in the ability of SrTreat to sorb Sr during its use, and to understand the causes of changes in the sorbing. After exposure to a simulated treated water for 99 h, the surface structure of the SrTreat was changed, and the percentage of sorbed Sr and the buffer capacity for protons decreased. When the amount of radioactive nuclides contained in the used SrTreat is calculated from the sorption data of the as received SrTreat.