Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 radionuclide therapy
radionuclide therapyOgawa, Kazuma*; Mizuno, Yoshiaki*; Washiyama, Koshin*; Shiba, Kazuhiro*; Takahashi, Naruto*; Kozaka, Takashi*; Watanabe, Shigeki; Shinohara, Atsushi*; Odani, Akira*
Nuclear Medicine and Biology, 42(11), p.875 - 879, 2015/11
Times Cited Count:27 Percentile:72.67(Radiology, Nuclear Medicine & Medical Imaging)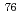 Br]bromo-
Br]bromo- -methyl-L-tyrosine, a novel tyrosine analog for positron emission tomography imaging of tumors
-methyl-L-tyrosine, a novel tyrosine analog for positron emission tomography imaging of tumorsOhshima, Yasuhiro; Hanaoka, Hirofumi*; Watanabe, Shigeki; Sugo, Yumi; Watanabe, Satoshi; Tominaga, Hideyuki*; Oriuchi, Noboru*; Endo, Keigo*; Ishioka, Noriko
Nuclear Medicine and Biology, 38(6), p.857 - 865, 2011/08
Times Cited Count:16 Percentile:47.57(Radiology, Nuclear Medicine & Medical Imaging) Cu
CuWatanabe, Shigeki; Watanabe, Satoshi; Liang, J. X.; Hanaoka, Hirofumi*; Endo, Keigo*; Ishioka, Noriko
Nuclear Medicine and Biology, 36(6), p.587 - 590, 2009/09
Times Cited Count:8 Percentile:27.84(Radiology, Nuclear Medicine & Medical Imaging) Re chelate-conjugated bisphosphonate for the development of new radiopharmaceuticals for bones
Re chelate-conjugated bisphosphonate for the development of new radiopharmaceuticals for bonesUehara, Tomoya*; Jin, Z. L.*; Ogawa, Kazuma*; Akizawa, Hiromichi*; Hashimoto, Kazuyuki; Nakayama, Morio*; Arano, Yasushi*
Nuclear Medicine and Biology, 34(1), p.79 - 87, 2007/01
Times Cited Count:27 Percentile:60.21(Radiology, Nuclear Medicine & Medical Imaging)In this study, a key factor affecting the pharmacokinetics of a chelate-conjugated BP was investigated to estimate the validity and the applicability of molecular design. Chemically inert and well-characterized [ Re]CpTR-Gly was conjugated with 3-amino-1-hydroxypropylidene-1,1-bisphosphonate and purified by HPLC to prepare [
Re]CpTR-Gly was conjugated with 3-amino-1-hydroxypropylidene-1,1-bisphosphonate and purified by HPLC to prepare [ Re]CpTR-Gly-APD. Plasma stability, plasma protein binding, hydroxyapatite (HA) binding and the pharmacokinetics of [
Re]CpTR-Gly-APD. Plasma stability, plasma protein binding, hydroxyapatite (HA) binding and the pharmacokinetics of [ Re]CpTR-Gly-APD were compared with those of
Re]CpTR-Gly-APD were compared with those of  Re 1-hydroxyethylidene-1,1-diphosphonate (HEDP). The HPLC-purified [
Re 1-hydroxyethylidene-1,1-diphosphonate (HEDP). The HPLC-purified [ Re]CpTR-Gly-APD showed higher plasma stability, higher HA binding, higher bone accumulation and lower plasma protein binding than did
Re]CpTR-Gly-APD showed higher plasma stability, higher HA binding, higher bone accumulation and lower plasma protein binding than did  Re -HEDP. Although
Re -HEDP. Although  Re -HEDP possessed HA binding and bone accumulation similar to those of [
Re -HEDP possessed HA binding and bone accumulation similar to those of [ Re]CpTR-Gly-APD, the specific activity of
Re]CpTR-Gly-APD, the specific activity of  Re -labeled BPs was found to play a crucial role in bone accumulation and blood clearance. Thus, the molecular design of chelate-conjugated BP would be useful for the development of bone-seeking radiopharmaceuticals with a variety of radionuclides by selecting chelating molecules that provide high specific activities.
Re -labeled BPs was found to play a crucial role in bone accumulation and blood clearance. Thus, the molecular design of chelate-conjugated BP would be useful for the development of bone-seeking radiopharmaceuticals with a variety of radionuclides by selecting chelating molecules that provide high specific activities.
Ogawa, Kazuma*; Mukai, Takahiro*; Arano, Yasushi*; Otaka, Akira*; Ueda, Masashi*; Uehara, Tomoya*; Magata, Yasuhiro*; Hashimoto, Kazuyuki; Saji, Hideo*
Nuclear Medicine and Biology, 33(4), p.513 - 520, 2006/05
Times Cited Count:57 Percentile:80.60(Radiology, Nuclear Medicine & Medical Imaging)To develop a radiopharmaceutical for the palliation of painful bone metastases based on the concept of bifunctional radiopharmaceuticals, we synthesized a bisphosphonate derivative labeled with rhenium-186 ( Re) that contains a hydroxyl group at the central carbon of its bisphosphonate structure and attached a stable
Re) that contains a hydroxyl group at the central carbon of its bisphosphonate structure and attached a stable  Re-MAMA chelate to the amino group of a 4-amino-butylidene-bisphosphonate derivative,
Re-MAMA chelate to the amino group of a 4-amino-butylidene-bisphosphonate derivative,  Re-MAMA-HBP, and investigated the effect of a hydroxyl group at the central carbon of its bisphosphonate structure on the affinity for hydroxyapatite and biodistribution by conducting a comparative study with
Re-MAMA-HBP, and investigated the effect of a hydroxyl group at the central carbon of its bisphosphonate structure on the affinity for hydroxyapatite and biodistribution by conducting a comparative study with  Re-MAMA-BP.
Re-MAMA-BP.  Re-MAMA-HBP was prepared by a reaction with
Re-MAMA-HBP was prepared by a reaction with  ReO
ReO
 and SnCl
and SnCl in citrate buffer after the deprotection of trityl groups of Tr-MAMA-HBP. After reversed phase HPLC,
in citrate buffer after the deprotection of trityl groups of Tr-MAMA-HBP. After reversed phase HPLC,  Re-MAMA-HBP had a radiochemical purity of over 95 %. Compared with
Re-MAMA-HBP had a radiochemical purity of over 95 %. Compared with  Re-MAMA-BP,
Re-MAMA-BP,  Re-MAMA-HBP showed a greater affinity for hydroxyapatite beads in vitro and accumulated a significantly higher level in the femur in vivo. Thus, the introduction of a hydroxyl group into
Re-MAMA-HBP showed a greater affinity for hydroxyapatite beads in vitro and accumulated a significantly higher level in the femur in vivo. Thus, the introduction of a hydroxyl group into  Re complex-conjugated bisphosphonates would be effective in enhancing accumulation in bone. These findings provide useful information on the design of bone-seeking therapeutic radiopharmaceuticals.
Re complex-conjugated bisphosphonates would be effective in enhancing accumulation in bone. These findings provide useful information on the design of bone-seeking therapeutic radiopharmaceuticals.
Uehara, Tomoya*; Koike, Miho*; Nakata, Hideo*; Miyamoto, Shigehiko*; Motoishi, Shoji; Hashimoto, Kazuyuki; Oku, Naoto*; Nakayama, Morio*; Arano, Yasushi*
Nuclear Medicine and Biology, 30(3), p.327 - 334, 2003/04
Times Cited Count:20 Percentile:49.32(Radiology, Nuclear Medicine & Medical Imaging)no abstracts in English