Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Duarte, A. G.*; Katata, Genki; Hoshika, Yasutomo*; Hossain, M.*; Kreuzwieser, J.*; Arneth, A.*; Ruehr, N. K.*
Journal of Plant Physiology, 205, p.57 - 66, 2016/10
Times Cited Count:45 Percentile:83.58(Plant Sciences)The frequency and intensity of climatic extremes, such as heat waves, are predicted to increase globally, with severe implications for terrestrial carbon and water cycling. Temperatures may rise above critical thresholds that allow trees to function optimally, with unknown long-term consequences for forest ecosystems. In this context, we investigated how photosynthetic traits and the water balance in Douglasfir are affected by exposure to three heat waves. Photosynthetic carboxylation efficiency was mostly unaffected, but electron transport and photosynthetic rates under saturating light were strongly influenced by the heat waves, with lagging limitations on photosynthesis still being observed six weeks after the last heat wave. We also observed lingering heat-induced inhibitions on transpiration, minimum stomatal conductance, and nighttime stomatal conductance. Results from the stomatal models used to calculate minimum stomatal conductance were similar to gs-night and indicated changes in leaf morphology, such as stomatal occlusions and alterations in epicuticular wax. Our results show Douglas-fir's ability to restrict water loss following heat stress, but at the price of reduced photosynthetic performance. Such limitations indicate potential long-term restrictions that heat waves can impose on tree development and functioning under extreme climatic conditions.
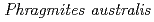 (Cav.) Trin. ex Steud.] at steady state under constant high-salt conditions
(Cav.) Trin. ex Steud.] at steady state under constant high-salt conditionsFujimaki, Shu; Maruyama, Teppei*; Suzui, Nobuo; Kawachi, Naoki; Miwa, Eitaro*; Higuchi, Kyoko*
Plant & Cell Physiology, 56(5), p.943 - 950, 2015/05
Times Cited Count:32 Percentile:72.88(Plant Sciences)We analyzed the directions and rates of translocation of sodium ions (Na ) within tissues of a salt-tolerant plant, common reed, and a salt-sensitive plant, rice, under constant high-salt conditions using radioactive
) within tissues of a salt-tolerant plant, common reed, and a salt-sensitive plant, rice, under constant high-salt conditions using radioactive  Na tracer and a positron-emitting tracer imaging system (PETIS). First, the test plants were incubated in a nutrient solution containing 50 mM NaCl and a trace level of
Na tracer and a positron-emitting tracer imaging system (PETIS). First, the test plants were incubated in a nutrient solution containing 50 mM NaCl and a trace level of  Na for 24 h (feeding step). Then the original solution was replaced with a fresh solution containing 50 mM NaCl but no
Na for 24 h (feeding step). Then the original solution was replaced with a fresh solution containing 50 mM NaCl but no  Na, in which the test plants remained for over 48 h (chase step). Non-invasive visualization of
Na, in which the test plants remained for over 48 h (chase step). Non-invasive visualization of  Na movement in the test plants was conducted during feeding and chase steps with PETIS. Our results revealed that
Na movement in the test plants was conducted during feeding and chase steps with PETIS. Our results revealed that  Na was absorbed in the roots of common reed, but not transported to the upper shoot beyond the shoot base. During the chase step, a basal-to-distal movement of
Na was absorbed in the roots of common reed, but not transported to the upper shoot beyond the shoot base. During the chase step, a basal-to-distal movement of  Na was detected within the root tissue with a velocity of approximately 0.5 cm h
Na was detected within the root tissue with a velocity of approximately 0.5 cm h . On the other hand,
. On the other hand,  Na that absorbed in the roots of rice was continuously translocated to the whole shoot. We concluded that the basal roots and the shoot base of common reed have constitutive functions of Na
Na that absorbed in the roots of rice was continuously translocated to the whole shoot. We concluded that the basal roots and the shoot base of common reed have constitutive functions of Na exclusion only in the direction of root tips, even under constant high-salt conditions. This function may apparently contribute to the low Na
exclusion only in the direction of root tips, even under constant high-salt conditions. This function may apparently contribute to the low Na concentration in the upper shoot and high salt tolerance of common reed.
concentration in the upper shoot and high salt tolerance of common reed.
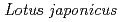 nodules
nodulesHakoyama, Tsuneo*; Oi, Ryo*; Hazuma, Kazuya*; Suga, Eri*; Adachi, Yuka*; Kobayashi, Mayumi*; Akai, Rie*; Sato, Shusei*; Fukai, Eigo*; Tabata, Satoshi*; et al.
Plant Physiology, 160(2), p.897 - 905, 2012/10
Times Cited Count:31 Percentile:67.21(Plant Sciences)
Nakasone, Akari*; Fujiwara, Masayuki*; Fukao, Yoichiro*; Biswas, K.; Rahman, A.*; Yamada, Maki*; Narumi, Issei; Uchimiya, Hirofumi*; Ono, Yutaka
Plant Physiology, 160(1), p.93 - 105, 2012/09
Times Cited Count:13 Percentile:38.37(Plant Sciences)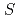 -transferase gene in cyclamen
-transferase gene in cyclamenKitamura, Satoshi; Akita, Yusuke; Ishizaka, Hiroshi*; Narumi, Issei; Tanaka, Atsushi
Journal of Plant Physiology, 169(6), p.636 - 642, 2012/04
Times Cited Count:87 Percentile:91.36(Plant Sciences)In order to identify the anthocyanin-related GST in cyclamen, four candidates of GSTs (CkmGST1 to CkmGST4) were isolated. Phylogenetic analysis indicated that CkmGST3 was closely related to PhAN9, an anthocyanin-related GST of petunia. Expression analysis at different developmental stages of petals revealed that CkmGST3 was strongly expressed in paler pigmented petals than in fully pigmented petals, in contrast to the constitutive expression of the other three candidates during petal development. This expression pattern of CkmGST3 was correlated with those of other anthocyaninbiosynthetic genes such as CkmF3'5'H and CkmDFR2. Molecular complementation of Arabidopsis 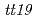 , a knockout mutant of an anthocyanin-related GST gene, demonstrated that CkmGST3 could complement the anthocyanin-less phenotype of
, a knockout mutant of an anthocyanin-related GST gene, demonstrated that CkmGST3 could complement the anthocyanin-less phenotype of 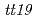 . Transgenic plants that expressed the other three CkmGSTs did not show anthocyanin accumulation. These results indicate CkmGST3 functions in anthocyanin accumulation in cyclamen.
. Transgenic plants that expressed the other three CkmGSTs did not show anthocyanin accumulation. These results indicate CkmGST3 functions in anthocyanin accumulation in cyclamen.
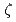 , AtRev1 and AtPol
, AtRev1 and AtPol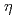 in UV light-induced mutagenesis in Arabidopsis
in UV light-induced mutagenesis in Arabidopsis![$$^{1[W]}$$](/search/images/ERPHWMK1K3OX0JA.gif)
Nakagawa, Mayu*; Takahashi, Shinya*; Tanaka, Atsushi; Narumi, Issei; Sakamoto, Ayako
Plant Physiology, 155(1), p.414 - 420, 2011/01
Times Cited Count:15 Percentile:41.13(Plant Sciences)no abstracts in English
Fujimaki, Shu; Suzui, Nobuo; Ishioka, Noriko; Kawachi, Naoki; Ito, Sayuri; Chino, Mitsuo*; Nakamura, Shinichi*
Plant Physiology, 152(4), p.1796 - 1806, 2010/02
Times Cited Count:217 Percentile:98.09(Plant Sciences)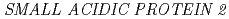 potentially mediates the response to synthetic auxin, 2,4-dichlorophenoxyacetic acid, in
potentially mediates the response to synthetic auxin, 2,4-dichlorophenoxyacetic acid, in 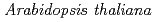
Nakasone, Akari; Yamada, Maki*; Kiyosue, Tomohiro*; Narumi, Issei; Uchimiya, Hirofumi*; Ono, Yutaka
Journal of Plant Physiology, 166(12), p.1307 - 1313, 2009/08
Times Cited Count:6 Percentile:17.74(Plant Sciences) Fe translocation in barley as monitored by a Positron-Emitting Tracer Imaging System (PETIS); Evidence for the direct translocation of Fe from roots to young leaves via phloem
Fe translocation in barley as monitored by a Positron-Emitting Tracer Imaging System (PETIS); Evidence for the direct translocation of Fe from roots to young leaves via phloemTsukamoto, Takashi*; Nakanishi, Hiromi*; Uchida, Hiroshi*; Watanabe, Satoshi; Matsuhashi, Shimpei; Mori, Satoshi*; Nishizawa, Naoko*
Plant & Cell Physiology, 50(1), p.48 - 57, 2009/01
Times Cited Count:96 Percentile:90.14(Plant Sciences)Okamoto, Takashi*; Tsurumi, Seiji*; Shibasaki, Kyohei*; Obana, Yoshimi*; Takaji, Hironori*; Ono, Yutaka; Rahman, A.*
Plant Physiology, 146(4), p.1651 - 1662, 2008/04
Times Cited Count:51 Percentile:76.19(Plant Sciences)The Arabidopsis seedlings grown horizontally on a dialysis membrane-covered agar plate encountered adequate mechanical impedance as the roots showed characteristic ethylene phenotypes: 2-fold reduction in root growth, increase in root diameter, decrease in cell elongation, and ectopic root hair formation. The root phenotype characterization of various mutants having altered response to ethylene biosynthesis or signaling, the effect of ethylene inhibitors on mechanically impeded roots, and transcription profiling of the ethylene-responsive genes led us to conclude that enhanced ethylene response plays a primary role in changing root morphology and development during mechanical impedance. Further, the differential sensitivity of horizontally and vertically grown roots toward exogenous ethylene suggested that ethylene signaling plays a critical role in enhancing the ethylene response. We subsequently demonstrated that the enhanced ethylene response also affects the auxin response in roots. Taken together, our results provide a new insight into the role of ethylene in changing root morphology during mechanical impedance.
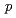 -chlorophenoxyisobutyric acid reveals that
-chlorophenoxyisobutyric acid reveals that 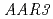 , a gene encoding a DCN1-like protein, regulates responses to the synthetic auxin 2,4-dichlorophenoxyacetic acid in Arabidopsis roots
, a gene encoding a DCN1-like protein, regulates responses to the synthetic auxin 2,4-dichlorophenoxyacetic acid in Arabidopsis rootsBiswas, K. K.*; Oura, Chiharu*; Higuchi, Kanako*; Miyazaki, Yuji*; Nguyen, V. V.*; Rahman, A.*; Uchimiya, Hirofumi*; Kiyosue, Tomohiro*; Koshiba, Tomokazu*; Tanaka, Atsushi; et al.
Plant Physiology, 145(3), p.773 - 785, 2007/11
Times Cited Count:42 Percentile:68.25(Plant Sciences)We screened mutants for root growth resistance to a putative antiauxin, PCIB, which inhibits auxin action by interfering the upstream auxin signaling events. Eleven PCIB-resistant mutants were obtained. Genetic mapping indicates that the mutations are located in at least 5 independent loci including two known auxin-related loci, 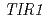 and
and 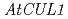 .
. 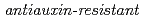 mutants (
mutants (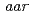 s)
s) 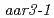 ,
, 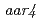 and
and 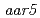 were also resistant to 2,4-D as shown by a root growth assay. Positional cloning of
were also resistant to 2,4-D as shown by a root growth assay. Positional cloning of 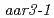 revealed that the
revealed that the 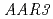 gene encodes a protein with a domain of unknown function (DUF298), which has not previously been implicated in auxin signaling. The protein has a putative nuclear localization signal and shares homology with the DCN-1 protein through the DUF298 domain. The results also indicate that PCIB can facilitate the identification of factors involved in auxin or auxin-related signaling.
gene encodes a protein with a domain of unknown function (DUF298), which has not previously been implicated in auxin signaling. The protein has a putative nuclear localization signal and shares homology with the DCN-1 protein through the DUF298 domain. The results also indicate that PCIB can facilitate the identification of factors involved in auxin or auxin-related signaling.
Takahashi, Shinya*; Sakamoto, Ayako; Tanaka, Atsushi; Shimizu, Kikuo*
Plant Physiology, 145(3), p.1052 - 1060, 2007/11
Times Cited Count:17 Percentile:39.71(Plant Sciences)To clarify the functions of AtREV1 protein, we expressed it in E. coli and purified it. The deoxynucleotidyl transferase activity of the recombinant AtREV1 was examined in a primer extension assay 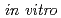 . The recombinant AtREV1 transferred one or two nucleotides to the primer end. Especially, it efficiently inserted dCMP regardless of the opposite base. AtREV1 also inserted a dCMP opposite the apurinic/apyrimidinic (AP) sites, which are physiologically generated or induced by various DNA-damaging agents. However, AtREV1 had no insertion activities against UV-inducible DNA lesions. Although the substrate specificity of AtREV1 was rather narrow in the presence of magnesium ion, it widened in the presence of manganese ion. These results suggest that AtREV1 serves as a deoxycytidyl transferase in plant cells.
. The recombinant AtREV1 transferred one or two nucleotides to the primer end. Especially, it efficiently inserted dCMP regardless of the opposite base. AtREV1 also inserted a dCMP opposite the apurinic/apyrimidinic (AP) sites, which are physiologically generated or induced by various DNA-damaging agents. However, AtREV1 had no insertion activities against UV-inducible DNA lesions. Although the substrate specificity of AtREV1 was rather narrow in the presence of magnesium ion, it widened in the presence of manganese ion. These results suggest that AtREV1 serves as a deoxycytidyl transferase in plant cells.
Dong, M. A.*; Bufford, J. L.*; Ono, Yutaka; Church, K.*; Dau, M. Q.*; Michels, K.*; Haughton, M.*; Tallman, G.*
Plant Physiology, 145(2), p.367 - 377, 2007/10
Times Cited Count:3 Percentile:8.87(Plant Sciences)Cultured guard cell protoplasts (GCP) of Nicotiana glauca, tree tobacco, comprise a novel system for investigating the cell signaling mechanisms that lead to acquired thermotolerance and thermoinhibition. At 32  C in a medium containing an auxin and a cytokinin, GCP expand, regenerate cell walls, dedifferentiate, and divide. At 38
C in a medium containing an auxin and a cytokinin, GCP expand, regenerate cell walls, dedifferentiate, and divide. At 38  C, GCP acquire thermotolerance, but their expansion is limited and they neither regenerate walls nor re-enter the cell cycle. Protoplasts were transformed with the fusion gene of BA auxin-responsive promoter and green fluorescent protein (GFP) gene. Heat suppressed auxin-mediated activation of BA. Heat-stressed cells accumulate reactive oxygen species, and H
C, GCP acquire thermotolerance, but their expansion is limited and they neither regenerate walls nor re-enter the cell cycle. Protoplasts were transformed with the fusion gene of BA auxin-responsive promoter and green fluorescent protein (GFP) gene. Heat suppressed auxin-mediated activation of BA. Heat-stressed cells accumulate reactive oxygen species, and H O
O suppresses auxin-responsive promoter activation. H
suppresses auxin-responsive promoter activation. H O
O did not suppress BA activation at 32
did not suppress BA activation at 32  C, nor did superoxide and H
C, nor did superoxide and H O
O scavengers prevent BA suppression at 38
scavengers prevent BA suppression at 38  C.
C.
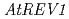 and
and 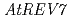 in translation synthesis
in translation synthesisTakahashi, Shinya; Sakamoto, Ayako; Sato, Shusei*; Kato, Tomohiko*; Tabata, Satoshi*; Tanaka, Atsushi
Plant Physiology, 138(2), p.870 - 881, 2005/06
Times Cited Count:53 Percentile:72.55(Plant Sciences)The error-prone DNA translation synthesis (error-prone TLS) has been well characterized in yeast and mammalians, but not in higher plants. Recent finding of an 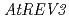 -disrupted mutant (
-disrupted mutant (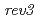 ) in Arabidopsis suggested that the error-prone TLS is significant for tolerance to DNA damages in higher plant. To clarify the details of the error-prone TLS in higher plants, we analyzed the T-DNA inserted Arabidopsis mutants defective in
) in Arabidopsis suggested that the error-prone TLS is significant for tolerance to DNA damages in higher plant. To clarify the details of the error-prone TLS in higher plants, we analyzed the T-DNA inserted Arabidopsis mutants defective in 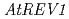 or
or 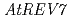 genes, which are thought to be involved in the error-prone TLS system. The
genes, which are thought to be involved in the error-prone TLS system. The 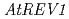 mutant (
mutant (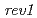 ) was sensitive to UV-B and cisplatin. The
) was sensitive to UV-B and cisplatin. The 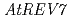 mutant (
mutant ( ) was sensitive to long-term UV-B and cisplatin. These results suggest TLS mechanism exists in a higher plant and show that
) was sensitive to long-term UV-B and cisplatin. These results suggest TLS mechanism exists in a higher plant and show that 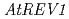 and
and 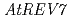 have important roles in tolerating exposure to DNA-damaging agents, but their function might be redundant.
have important roles in tolerating exposure to DNA-damaging agents, but their function might be redundant.
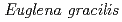
Hayashi, Hirotaka; Narumi, Issei; Wada, Seiichi; Kikuchi, Masahiro; Furuta, Masakazu*; Uehara, Kaku*; Watanabe, Hiroshi*
Journal of Plant Physiology, 161(10), p.1101 - 1106, 2004/10
Times Cited Count:11 Percentile:24.45(Plant Sciences)The resistance of 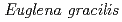 strains Z (wild type) and SM-ZK (chloroplast-deficient mutant) to ionizing radiation was investigated. The colony forming ability of
strains Z (wild type) and SM-ZK (chloroplast-deficient mutant) to ionizing radiation was investigated. The colony forming ability of 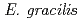 strain Z was higher than that of strain SM-ZK after
strain Z was higher than that of strain SM-ZK after  -irradiation. For both strains, the resistance of light-grown cells was higher than that of dark-grown cells, suggesting that the light conditions during the culture contribute to the radiation resistance of
-irradiation. For both strains, the resistance of light-grown cells was higher than that of dark-grown cells, suggesting that the light conditions during the culture contribute to the radiation resistance of 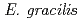 . The comet assay showed that the ability of rejoining DNA double-strand breaks (dsb) was much higher in the light-grown cells. These results suggest that
. The comet assay showed that the ability of rejoining DNA double-strand breaks (dsb) was much higher in the light-grown cells. These results suggest that 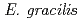 possesses a light-induced repair system to cope with DNA dsb.
possesses a light-induced repair system to cope with DNA dsb.
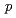 -chlorophenoxyisobutyric acid impairs auxin response in arabidopsis root
-chlorophenoxyisobutyric acid impairs auxin response in arabidopsis rootOno, Yutaka; Oura, Chiharu*; Rahman, A.; Aspuria, E. T.; Hayashi, Kenichiro*; Tanaka, Atsushi; Uchimiya, Hirofumi*
Plant Physiology, 133(3), p.1135 - 1147, 2003/11
Times Cited Count:142 Percentile:92.90(Plant Sciences)PCIB (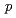 -chlorophenoxyisobutyric acid) is known as a putative antiauxin and is widely used to inhibit auxin action, although the mechanism of PCIB-mediated inhibition of auxin action is not characterized very well at molecular level. In the present work, we showed that PCIB inhibited BA::GUS expression induced by IAA, 2,4-D and NAA. PCIB also inhibited auxin dependent DR5::GUS expression. RNA hybridization and quantitative RT-PCR analyses suggested that PCIB reduced auxin-induced accumulation of transcripts of
-chlorophenoxyisobutyric acid) is known as a putative antiauxin and is widely used to inhibit auxin action, although the mechanism of PCIB-mediated inhibition of auxin action is not characterized very well at molecular level. In the present work, we showed that PCIB inhibited BA::GUS expression induced by IAA, 2,4-D and NAA. PCIB also inhibited auxin dependent DR5::GUS expression. RNA hybridization and quantitative RT-PCR analyses suggested that PCIB reduced auxin-induced accumulation of transcripts of 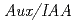 genes. In addition, PCIB relieved the reduction of GUS activity in
genes. In addition, PCIB relieved the reduction of GUS activity in 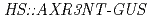 transgenic line in which auxin inhibits GUS activity by promoting degradation of the AXR3NT-GUS fusion protein. Physiological analysis revealed that PCIB inhibited lateral root production, gravitropic response of roots and growth of primary roots. These results suggest that PCIB impairs auxin signaling pathway by regulating Aux/IAA protein stability, and thereby affects the auxin-regulated Arabidopsis root physiology.
transgenic line in which auxin inhibits GUS activity by promoting degradation of the AXR3NT-GUS fusion protein. Physiological analysis revealed that PCIB inhibited lateral root production, gravitropic response of roots and growth of primary roots. These results suggest that PCIB impairs auxin signaling pathway by regulating Aux/IAA protein stability, and thereby affects the auxin-regulated Arabidopsis root physiology.
Tanaka, Atsushi; Sakamoto, Ayako; Ishigaki, Yasuhito*; Nikaido, Osamu*; Sun, G.; Hase, Yoshihiro; Shikazono, Naoya; Tano, Shigemitsu; Watanabe, Hiroshi
Plant Physiology, 129(1), p.64 - 71, 2002/05
Times Cited Count:75 Percentile:82.54(Plant Sciences)no abstracts in English
Choe, S.*; Dilkes, B. P.*; Gregory, B. D.*; Ross, A. S.*; Yuan, H.*; Noguchi, Takahiro*; Fujioka, Shozo*; Takatsuto, Suguru*; Tanaka, Atsushi; Yoshida, Shigeo*; et al.
Plant Physiology, 119(3), p.897 - 907, 1999/03
Times Cited Count:205 Percentile:97.50(Plant Sciences)no abstracts in English