Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Micheau, C.; Ueda, Yuki; Akutsu, Kazuhiro*; Bourgeois, D.*; Motokawa, Ryuhei
Solvent Extraction and Ion Exchange, 41(2), p.221 - 240, 2023/02
Times Cited Count:4 Percentile:41.44(Chemistry, Multidisciplinary)Simonnet, M.; Sasaki, Yuji; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 41(7), p.857 - 867, 2023/00
Times Cited Count:4 Percentile:41.44(Chemistry, Multidisciplinary)Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Solvent Extraction and Ion Exchange, 40(6), p.620 - 640, 2022/00
Times Cited Count:2 Percentile:12.88(Chemistry, Multidisciplinary)Owing to the chemical behavior of trivalent lanthanide and actinide ions with similar ionic radii, realizing this separation is still challenging. All lanthanides, Am, and Cm can be extracted using diglycolamide (DGA), and relatively high An/Ln separation efficiencies have been obtained using diethylenetriamine-triacetic-bisamide (DTBA). To improve the previous results as well as the separation conditions, we used organic acids for pH adjustment. The advantages of this modification included low HNO , DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
, DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
 -diketone extractant and a neutral ligand
-diketone extractant and a neutral ligandMatsumiya, Masahiko*; Nomizu, Daiki*; Tsuchida, Yusuke*; Sasaki, Yuji
Solvent Extraction and Ion Exchange, 39(7), p.764 - 784, 2021/00
Times Cited Count:6 Percentile:30.15(Chemistry, Multidisciplinary)We investigated the solvent extraction of four rare earth (RE) elements (Pr, Nd, Tb, and Dy) from Nd-Fe-B magnets using mixtures of 1-(2-thienyl)-4,4,4,-trifluoro-1,3-butanedione (Htta) or 4,4,4-trifluoro-1-phenyl-1,3-butanedione (Hbfa) chelating extractants and tri-n-octylphosphine oxide (TOPO) neutral ligand in phosphonium based ionic liquids. A synergistic effect was observed for the extraction of the RE elements with the combination of extractant and neutral ligand. The separation of Tb(III) and Dy(III) from other RE(III) components was performed with seven extraction cycles.
 from nitrate media; Comparison with a conventional organic phosphate
from nitrate media; Comparison with a conventional organic phosphateUeda, Yuki; Kikuchi, Kei*; Tokunaga, Kohei; Sugita, Tsuyoshi; Aoyagi, Noboru; Tanaka, Kazuya; Okamura, Hiroyuki
Solvent Extraction and Ion Exchange, 39(5-6), p.491 - 511, 2021/00
Times Cited Count:4 Percentile:19.51(Chemistry, Multidisciplinary)no abstracts in English
Toigawa, Tomohiro; Tsubata, Yasuhiro; Kai, Takeshi; Furuta, Takuya; Kumagai, Yuta; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 39(1), p.74 - 89, 2021/00
Times Cited Count:2 Percentile:7.36(Chemistry, Multidisciplinary)Absorbed-dose estimation is essential for evaluation of the radiation feasibility of minor-actinide-separation processes. We propose a dose-evaluation method based on radiation permeability, with comparisons of heterogeneous structures seen in the solvent-extraction process, such as emulsions forming in the mixture of the organic and aqueous phases. A demonstration of radiation-energy-transfer simulation is performed with a focus on the minor-actinide-recovery process from high-level liquid waste with the aid of the Monte Carlo radiation-transport code PHITS. The simulation results indicate that the dose absorbed by the extraction solvent from alpha ray depends upon the emulsion structure, and that from beta and gamma ray depends upon the mixer-settler-apparatus size. Non-negligible contributions of well-permeable gamma rays were indicated in terms of the plant operation of the minor-actinide-separation process.
Simonnet, M.; Suzuki, Shinichi; Miyazaki, Yuji*; Kobayashi, Toru; Yokoyama, Keiichi; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 38(4), p.430 - 440, 2020/00
Times Cited Count:23 Percentile:68.08(Chemistry, Multidisciplinary) -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(7), p.489 - 499, 2019/11
Times Cited Count:24 Percentile:63.88(Chemistry, Multidisciplinary)A continuous counter-current experiment to separate minor actinides (MAs: Am and Cm) was performed with  -hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm
-hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm ) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be
) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be  99% by optimizing separation conditions.
99% by optimizing separation conditions.
 solution; Comparison with tri-
solution; Comparison with tri- -butyl phosphate
-butyl phosphateUeda, Yuki; Kikuchi, Kei; Sugita, Tsuyoshi; Motokawa, Ryuhei
Solvent Extraction and Ion Exchange, 37(5), p.347 - 359, 2019/07
Times Cited Count:10 Percentile:34.62(Chemistry, Multidisciplinary)We have newly-designed fluorous phosphate(TFP) for the effective Zr(IV) ion extractant as an alternative extractant against the conventional organic phosphate, tri- -butyl phosphate(TBP). Zr(IV) ion extraction system using the TBP has many problems such as the formation of the third phase during liquid-liquid extraction. Here, we develop the fluorous extraction system based on the TFP-perfluorohexane for the Zr(IV) ion extraction to improve the Zr(IV) ion extraction system with an effective extractability and without the third phase formation. Our main findings were that the significant high extraction performance of the TFP for Zr(IV) ion as compared with TBP, and the origins of the high extraction performance of the TFP are related to the water and HNO
-butyl phosphate(TBP). Zr(IV) ion extraction system using the TBP has many problems such as the formation of the third phase during liquid-liquid extraction. Here, we develop the fluorous extraction system based on the TFP-perfluorohexane for the Zr(IV) ion extraction to improve the Zr(IV) ion extraction system with an effective extractability and without the third phase formation. Our main findings were that the significant high extraction performance of the TFP for Zr(IV) ion as compared with TBP, and the origins of the high extraction performance of the TFP are related to the water and HNO contents in the fluorous phase and the stability of the complex, Zr(No
contents in the fluorous phase and the stability of the complex, Zr(No )
) (TFP)
(TFP) .
.
 -tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cell
-tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya; Hotoku, Shinobu; Kawasaki, Tomohiro*; Sagawa, Hiroshi*; Tsutsui, Nao; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(1), p.27 - 37, 2019/00
Times Cited Count:25 Percentile:65.19(Chemistry, Multidisciplinary)A continuous counter-current experiment using TDdDGA was performed using mixer-settler extractors installed in a hot cell. Nitric acid containing minor actinides (MAs: Am and Cm), rare earths (REs: Y, La, Nd, and Eu), and other fission products (Sr, Cs, Zr, Mo, Ru, Rh, and Pd) was fed to the extractor. TDdDGA effectively extracted MAs and REs from the feed, while other fission products were barely extracted. The extracted MAs and REs were back-extracted by bringing them in contact with 0.02 mol/dm nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were
nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were  98% and
98% and  86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
Simonnet, M.; Miyazaki, Yuji*; Suzuki, Shinichi; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 37(1), p.81 - 95, 2019/00
Times Cited Count:9 Percentile:28.32(Chemistry, Multidisciplinary) ,
, -di(2-ethylhexyl)octanamide from nitric acid medium
-di(2-ethylhexyl)octanamide from nitric acid mediumTsutsui, Nao; Ban, Yasutoshi; Sagawa, Hiroshi; Ishii, Sho; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 35(6), p.439 - 449, 2017/08
Times Cited Count:7 Percentile:20.91(Chemistry, Multidisciplinary)Solvent extraction of uranium from a nitric acid medium was performed with  ,
, -di(2-ethylhexyl)octanamide (DEHOA) by a single-stage batch method, and the distribution ratio equation of U(VI) was derived as
-di(2-ethylhexyl)octanamide (DEHOA) by a single-stage batch method, and the distribution ratio equation of U(VI) was derived as 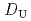 = 1.1
= 1.1![$$[rm NO^{-}_{3}]^{1.6}_{rm aq}[{rm DEHOA}]^{2}_{rm org}$$](/search/images/ERNVY2TNEBHE4XT1FV4V44ZTPVOV24ZRFY1H0X11LRZG0IDBOF4VW402OJWSARCFJBHUC5K3LZ3TE5K5PNOHE1JAN3ZGO5JE.gif) . Furthermore, the nitric acid distribution was also evaluated, and the distribution ratio equation
. Furthermore, the nitric acid distribution was also evaluated, and the distribution ratio equation 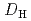 = 0.12
= 0.12![$$[rm H^{+}]^{0.76}_{rm aq}[{rm DEHOA_{rm Free}}]_{rm H}$$](/search/images/ERNVY2TNEBEF24ZLPVOV24ZQFY1TM5K5PNOHE1JAMFYX0W11LRZG0ICEIVEE4QK5PNOHE1JAIZZGKZL3PVOV4402OJWSASD3EQ.gif) was obtained. Batch experiments to evaluate the time dependence of U(VI) extraction and the U(VI) loading capacity of DEHOA were also performed. It was revealed that U(VI) extraction by DEHOA reached an equilibrium state within a few minutes, and the loading capacity was 0.71 mol/dm
was obtained. Batch experiments to evaluate the time dependence of U(VI) extraction and the U(VI) loading capacity of DEHOA were also performed. It was revealed that U(VI) extraction by DEHOA reached an equilibrium state within a few minutes, and the loading capacity was 0.71 mol/dm (M) when the concentrations of DEHOA and nitric acid were 1.5 and 3.0 M, respectively.
(M) when the concentrations of DEHOA and nitric acid were 1.5 and 3.0 M, respectively.
Motokawa, Ryuhei; Endo, Hitoshi*; Nagao, Michihiro*; Heller, W. T.*
Solvent Extraction and Ion Exchange, 34(5), p.399 - 406, 2016/07
Times Cited Count:5 Percentile:17.92(Chemistry, Multidisciplinary) -dialkylamides (DEHDMPA and DEHBA) in mixer-settler extractors
-dialkylamides (DEHDMPA and DEHBA) in mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 34(1), p.37 - 47, 2016/01
Times Cited Count:10 Percentile:32.43(Chemistry, Multidisciplinary)The extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) and
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) and  -di(2-ethylhexyl)butanamide (DEHBA) for Np(V) and Np(VI) were studied by a batch method using various nitrate ion concentrations. The distribution ratios of Np(VI) obtained with DEHDMPA and DEHBA exceeded unity when the nitrate ion concentration was
-di(2-ethylhexyl)butanamide (DEHBA) for Np(V) and Np(VI) were studied by a batch method using various nitrate ion concentrations. The distribution ratios of Np(VI) obtained with DEHDMPA and DEHBA exceeded unity when the nitrate ion concentration was  3 mol/L. DEHDMPA and DEHBA barely extracted Np(V), and the maximum distribution ratios were 0.4 and 0.2 when DEHDMPA and DEHBA were used as extractants, respectively. A continuous counter-current experiment was performed to evaluate the behavior of Np in a process comprising two cycles. The ratio of Np recovered to the U fraction and U-Pu fraction were 63.7% and 29.1%, respectively. The behavior of Np suggested that the valence state of Np changed from Np(V) to Np(IV) or Np(VI) after the 1st experimental cycle. The recoveries of U and Pu to the U fraction stream and the U-Pu fraction stream were 99.9% and 99.8%, respectively.
3 mol/L. DEHDMPA and DEHBA barely extracted Np(V), and the maximum distribution ratios were 0.4 and 0.2 when DEHDMPA and DEHBA were used as extractants, respectively. A continuous counter-current experiment was performed to evaluate the behavior of Np in a process comprising two cycles. The ratio of Np recovered to the U fraction and U-Pu fraction were 63.7% and 29.1%, respectively. The behavior of Np suggested that the valence state of Np changed from Np(V) to Np(IV) or Np(VI) after the 1st experimental cycle. The recoveries of U and Pu to the U fraction stream and the U-Pu fraction stream were 99.9% and 99.8%, respectively.
Sasaki, Yuji; Sugo, Yumi; Morita, Keisuke; Nash, K.*; Nash, K. L.*
Solvent Extraction and Ion Exchange, 33(7), p.625 - 641, 2015/10
Times Cited Count:112 Percentile:91.79(Chemistry, Multidisciplinary)The effect of alkyl substituents at amidic N atoms in diglycolamide (DGA) compounds on solvent extraction was investigated. The solubility in water and n-dodecane, loading capacity, and distribution ratio (D) of lanthanides and actinides for various DGA compounds were monitored. DGA derivatives with short alkyl chains, for example methyl and ethyl groups, are highly water soluble, while DGA derivatives with long alkyl chains are highly soluble in n-dodecane. The loading capacity correlats with their alkyl chain lengths. The results of masking effect and solubility tests indicate that TEDGA(tetraethyl-diglycolamide) is the best actinide masking agent among the water-soluble DGA derivatives. Actinide extractions were performed using ten DGA compounds in six diluents (nitrobenzene, 1,2-dichloroethane, 1-octanol, chloroform, toluene, and n-dodecane), in which it was observed that DGA derivatives with shorter alkyl chains show higher D values.
 -di(2-ethylhexyl)-2,2-dimetnylpropanamide (DEHDMPA) and
-di(2-ethylhexyl)-2,2-dimetnylpropanamide (DEHDMPA) and  -di(2-ethylhexyl)butanamide (DEHBA) using mixer-settler extractors
-di(2-ethylhexyl)butanamide (DEHBA) using mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Morita, Yasuji
Solvent Extraction and Ion Exchange, 32(4), p.348 - 364, 2014/05
Times Cited Count:11 Percentile:34.33(Chemistry, Multidisciplinary)Extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) for nitric acid, U(VI), and Pu(IV) were studied, and the distribution ratio equations were derived for each chemical species. A continuous counter-current experiment was performed using mixer-settler extractors with two types of monoamides, DEHDMPA and
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) for nitric acid, U(VI), and Pu(IV) were studied, and the distribution ratio equations were derived for each chemical species. A continuous counter-current experiment was performed using mixer-settler extractors with two types of monoamides, DEHDMPA and  -di(2-ethylhexyl)butanamide (DEHBA), as extractants. DEHDMPA exclusively extracted U from the feed, and the ratio of U recovered in the U fraction stream was 99.93%. Almost all Pu were extracted by DEHBA, and the recovery of Pu in the U-Pu fraction stream was 99.94%. Concentrations of U and Pu in mixer-settlers were calculated using a simulation code, which confirmed that the calculation was effective for estimating the U concentration in the U fraction stream, and the U and Pu concentrations in the U-Pu fraction stream.
-di(2-ethylhexyl)butanamide (DEHBA), as extractants. DEHDMPA exclusively extracted U from the feed, and the ratio of U recovered in the U fraction stream was 99.93%. Almost all Pu were extracted by DEHBA, and the recovery of Pu in the U-Pu fraction stream was 99.94%. Concentrations of U and Pu in mixer-settlers were calculated using a simulation code, which confirmed that the calculation was effective for estimating the U concentration in the U fraction stream, and the U and Pu concentrations in the U-Pu fraction stream.
Shimada, Asako; Sulakova, J.*; Yang, Y.*; Alexandratos, S.*; Nash, K. L.*
Solvent Extraction and Ion Exchange, 32(1), p.27 - 43, 2014/01
Times Cited Count:5 Percentile:15.89(Chemistry, Multidisciplinary)A tetramethylmalonamide-functionalized resin (TMMA resin) has been developed and investigated for its ability to separate trivalent, tetravalent, and hexavalent actinide elements. As a fundamental study of the extraction mechanism and to design a chromatographic separation scheme, distribution coefficients (
 ) for partitioning of Eu(III), Th(IV), U(VI), and Am(III) onto the resin from HNO
) for partitioning of Eu(III), Th(IV), U(VI), and Am(III) onto the resin from HNO , NaNO
, NaNO , HCl and NaCl solutions with and without organic acids and potential consistents of low-level radioactve waste have been determined. The competition between metal extraction and HNO
, HCl and NaCl solutions with and without organic acids and potential consistents of low-level radioactve waste have been determined. The competition between metal extraction and HNO extraction was confirmed by the extraction data and FT-IR spectra. Based on these fundamental experimental results, an extractive chromatographic scheme for actinide isolation was designed and demonstrated.
extraction was confirmed by the extraction data and FT-IR spectra. Based on these fundamental experimental results, an extractive chromatographic scheme for actinide isolation was designed and demonstrated.
 -di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractors
-di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Morita, Yasuji
Solvent Extraction and Ion Exchange, 31(6), p.590 - 603, 2013/09
Times Cited Count:12 Percentile:40.61(Chemistry, Multidisciplinary)The recovery of U and Pu from nitric acid using the monoamide extractant  -di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractors was calculated using a simulation code, and a continuous counter-current experiment using mixer-settler extractors was performed. The flow rate, stage number, and nitric acid concentration were chosen as the parameters for the calculation, and the appropriate experimental conditions for separating U from Pu were determined. The results of the continuous counter-current experiment showed that the percentages of U and Pu extracted using 1.5 mol/dm
-di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractors was calculated using a simulation code, and a continuous counter-current experiment using mixer-settler extractors was performed. The flow rate, stage number, and nitric acid concentration were chosen as the parameters for the calculation, and the appropriate experimental conditions for separating U from Pu were determined. The results of the continuous counter-current experiment showed that the percentages of U and Pu extracted using 1.5 mol/dm (M) DEHBA from 4 M nitric acid were
(M) DEHBA from 4 M nitric acid were  99.9% and 97.84%, respectively.
99.9% and 97.84%, respectively.
Wada, Go*; Ishihara, Ryo*; Miyoshi, Kazuyoshi*; Umeno, Daisuke*; Saito, Kyoichi*; Asai, Shiho; Yamada, Shinsuke*; Hirota, Hideyuki*
Solvent Extraction and Ion Exchange, 31(2), p.210 - 220, 2013/02
Times Cited Count:2 Percentile:11.59(Chemistry, Multidisciplinary)A crosslinked chelating porous sheet was prepared by cografting ethylene glycol dimethacrylate (EGDMA) with glycidyl methacrylate onto an electron-beam-irradiated porous polyethylene sheet, followed by the introduction of an iminodiacetate group. At a molar percentage of EGDMA of 1.0 mol%, the sheet exhibited a maximum dynamic binding capacity for copper ions of 0.93 mmol/g, while the equilibrium binding capacity remained the same (1.2 mmol/g) as that of a non-crosslinked chelating porous sheet. The crosslinking of the grafted chain causes copper ions to lower their diffusion rate along the sheet thickness driven by the gradient of the amount of copper ions adsorbed.
 -di(2-ethylhexyl)butanamide in continuous counter-current extraction with mixer-settler extractor
-di(2-ethylhexyl)butanamide in continuous counter-current extraction with mixer-settler extractorBan, Yasutoshi; Hotoku, Shinobu; Morita, Yasuji
Solvent Extraction and Ion Exchange, 30(2), p.142 - 155, 2012/02
Times Cited Count:8 Percentile:30.67(Chemistry, Multidisciplinary)The extraction properties of 1.5 mol/dm (M)
(M)  -di-(2-ethyl-hexyl)butanamide (DEHBA) diluted with
-di-(2-ethyl-hexyl)butanamide (DEHBA) diluted with  -dodecane toward U(VI) and Pu(IV) were studied using a single stage batch method, and the distribution ratios toward U(VI) and Pu(IV) were, respectively, obtained as follows:
-dodecane toward U(VI) and Pu(IV) were studied using a single stage batch method, and the distribution ratios toward U(VI) and Pu(IV) were, respectively, obtained as follows: 
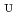 = 1.4[NO
= 1.4[NO
 ]
]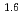 [DEHBA
[DEHBA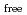 ]
] and
and 
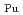 = 0.11[NO
= 0.11[NO
 ]
]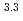 [DEHBA
[DEHBA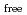 ]
] . A continuous counter-current experiment with 1.5 M DEHBA as an extractant was performed using mixer-settler extractors. The ratios of U(VI) and Pu(IV) extracted by 1.5 M DEHBA in the U-Pu extraction step were more than 99.9%. The extracted Pu(IV) was back-extracted using 0.67 M nitric acid, and more than 97% of Pu(IV) in the feed was recovered in the Pu fraction. The present results indicated that DEHBA works as an extractant for mutual separation of U(VI) and Pu(IV) by adjusting the nitric acid concentration without using Pu(IV) reductants.
. A continuous counter-current experiment with 1.5 M DEHBA as an extractant was performed using mixer-settler extractors. The ratios of U(VI) and Pu(IV) extracted by 1.5 M DEHBA in the U-Pu extraction step were more than 99.9%. The extracted Pu(IV) was back-extracted using 0.67 M nitric acid, and more than 97% of Pu(IV) in the feed was recovered in the Pu fraction. The present results indicated that DEHBA works as an extractant for mutual separation of U(VI) and Pu(IV) by adjusting the nitric acid concentration without using Pu(IV) reductants.