Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Sr using
Sr using  Sr/
Sr/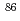 Sr isotope dilution method
Sr isotope dilution methodYanagisawa, Kayo*; Odashima, Mizuki*; Matsueda, Makoto; Furukawa, Makoto*; Takagai, Yoshitaka*
Talanta, 244, p.123442_1 - 123442_7, 2022/07
Times Cited Count:5 Percentile:37.69(Chemistry, Analytical)The determination of a low concentration of  Sr was achieved by the combination of online SPE-ICP-QMS and ID method using
Sr was achieved by the combination of online SPE-ICP-QMS and ID method using  Sr/
Sr/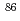 Sr ratio. No requirement of radioactive standard material and the preparation process of the calibration curve was in the quantification process and data acquisition can be in one-shot sample injection. The proposed method allowed the rapid (within 15 min/sample) quantification of
Sr ratio. No requirement of radioactive standard material and the preparation process of the calibration curve was in the quantification process and data acquisition can be in one-shot sample injection. The proposed method allowed the rapid (within 15 min/sample) quantification of  Sr in the presence of significant interferences such as isobaric
Sr in the presence of significant interferences such as isobaric  Zr and other elements. The LOD for
Zr and other elements. The LOD for  Sr was 5.6 Bq/L for a 10 mL injection and this could be improved by simply increasing the sample volume injected.
Sr was 5.6 Bq/L for a 10 mL injection and this could be improved by simply increasing the sample volume injected.
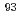 Zr determination with isotope dilution ICP-MS
Zr determination with isotope dilution ICP-MSAsai, Shiho; Hanzawa, Yukiko; Konda, Miki; Suzuki, Daisuke; Magara, Masaaki; Kimura, Takaumi; Ishihara, Ryo*; Saito, Kyoichi*; Yamada, Shinsuke*; Hirota, Hideyuki*
Talanta, 185, p.98 - 105, 2018/08
Times Cited Count:9 Percentile:30.63(Chemistry, Analytical)Estimating the risks associated with radiation from long-lived fission products (LLFP) in radioactive waste is essential to ensure the long-term safety of potential disposal sites. In this study, the amount of 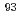 Zr, a LLFP, was determined by ICP-MS after separating Zr from a spent nuclear fuel solution using a microvolume anion-exchange cartridge (TEDA cartridge). The TEDA cartridge achieved highly selective separation of Zr regardless of its small bed volume of 0.08 cm
Zr, a LLFP, was determined by ICP-MS after separating Zr from a spent nuclear fuel solution using a microvolume anion-exchange cartridge (TEDA cartridge). The TEDA cartridge achieved highly selective separation of Zr regardless of its small bed volume of 0.08 cm . The time taken to complete the Zr separation was 1.2 min with a flow rate of 1.5 mL/min, which was 10 times faster than that for a conventional anion-exchange resin column. Almost all the other elements were removed, leading to accurate measurement of
. The time taken to complete the Zr separation was 1.2 min with a flow rate of 1.5 mL/min, which was 10 times faster than that for a conventional anion-exchange resin column. Almost all the other elements were removed, leading to accurate measurement of 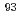 Zr. The result connects experimental value to theoretical prediction provided by ORIGEN2, which requires verification. With the measured value, we demonstrated that the theoretical value is reliable enough to estimate radiation risks.
Zr. The result connects experimental value to theoretical prediction provided by ORIGEN2, which requires verification. With the measured value, we demonstrated that the theoretical value is reliable enough to estimate radiation risks.
Do, V. K.; Yamamoto, Masahiko; Taguchi, Shigeo; Takamura, Yuzuru*; Surugaya, Naoki; Kuno, Takehiko
Talanta, 183, p.283 - 289, 2018/06
Times Cited Count:11 Percentile:37.19(Chemistry, Analytical)We develop a novel analytical method employing liquid electrode plasma optical emission spectrometry for measurement of total cesium in highly active liquid wastes. Limit of detection and limit of quantification are 0.005 mg/L and 0.02 mg/L, respectively. The method is validated and applied to the real samples.
 Pu/
Pu/ Pu in individual U-Pu mixed oxide particles by means of a combination of alpha spectrometry and ICP-MS
Pu in individual U-Pu mixed oxide particles by means of a combination of alpha spectrometry and ICP-MSEsaka, Fumitaka; Yasuda, Kenichiro; Suzuki, Daisuke; Miyamoto, Yutaka; Magara, Masaaki
Talanta, 165, p.122 - 127, 2017/04
Times Cited Count:20 Percentile:52.05(Chemistry, Analytical)The isotope ratios of  Pu/
Pu/ Pu,
Pu,  Pu/
Pu/ Pu,
Pu,  Pu/
Pu/ Pu, and
Pu, and 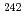 Pu/
Pu/ Pu were measured for individual Pu and U-Pu mixed oxide particles by a combination of alpha spectrometry and inductively coupled plasma mass spectrometry (ICP-MS). As a consequence, we were able to determine the
Pu were measured for individual Pu and U-Pu mixed oxide particles by a combination of alpha spectrometry and inductively coupled plasma mass spectrometry (ICP-MS). As a consequence, we were able to determine the  Pu/
Pu/ Pu,
Pu,  Pu/
Pu/ Pu, and
Pu, and 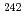 Pu/
Pu/ Pu isotope ratios with ICP-MS after particle dissolution and chemical separation of uranium, plutonium and americium with UTEVA resins. Furthermore,
Pu isotope ratios with ICP-MS after particle dissolution and chemical separation of uranium, plutonium and americium with UTEVA resins. Furthermore,  Pu/
Pu/ Pu isotope ratios were able to be calculated by using both the
Pu isotope ratios were able to be calculated by using both the  Pu/(
Pu/( Pu+
Pu+ Pu) activity ratios that had been measured through alpha spectrometry and the
Pu) activity ratios that had been measured through alpha spectrometry and the  Pu/
Pu/ Pu isotope ratios determined through ICP-MS. Therefore, the combined use of alpha spectrometry and ICP-MS is useful in determining plutonium isotope ratios, including
Pu isotope ratios determined through ICP-MS. Therefore, the combined use of alpha spectrometry and ICP-MS is useful in determining plutonium isotope ratios, including  Pu/
Pu/ Pu, in individual U-Pu mixed oxide particles.
Pu, in individual U-Pu mixed oxide particles.
Lee, C.-G.*; Suzuki, Daisuke; Esaka, Fumitaka; Magara, Masaaki; Song, K.*
Talanta, 141, p.92 - 96, 2015/08
Times Cited Count:16 Percentile:48.65(Chemistry, Analytical)Thermal ionization mass spectrometry (TIMS) with a continuous heating technique is known as an effective method for measuring the isotope ratio in trace amounts of uranium. In this study, the analytical performance of thermal ionization mass spectrometry with a continuous heating technique was investigated using a standard plutonium solution (SRM 947). The influence of the heating rate of the evaporation filament on the precision and accuracy of the isotope ratios was examined using a plutonium solution sample at the fg level. Changing the heating rate of the evaporation filament on samples ranging from 0.1 fg to 1000 fg revealed that the influence of the heating rate on the precision and accuracy of the isotope ratios was slight around the heating rate range of 100 to 250 mA/min. All of the isotope ratios of plutonium (SRM 947),  Pu/
Pu/ Pu,
Pu,  Pu/
Pu/ Pu,
Pu,  Pu/
Pu/ Pu and
Pu and 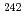 Pu/
Pu/ Pu, were measured down to sample amounts of 70 fg. The ratio of
Pu, were measured down to sample amounts of 70 fg. The ratio of  Pu/
Pu/ Pu was measured down to a sample amount of 0.1 fg, which corresponds to a PuO
Pu was measured down to a sample amount of 0.1 fg, which corresponds to a PuO particle with a diameter of 0.2
particle with a diameter of 0.2  m. Moreover, the signals of
m. Moreover, the signals of  Pu could be detected with a sample amount of 0.03 fg, which corresponds to the detection limit of
Pu could be detected with a sample amount of 0.03 fg, which corresponds to the detection limit of  Pu of 0.006 fg as estimated by the 3
Pu of 0.006 fg as estimated by the 3  criterion.
criterion.  Pu and
Pu and  Am formed by the decay of
Am formed by the decay of  Pu could be discriminated owing to the difference in the evaporation temperature. As a result,
Pu could be discriminated owing to the difference in the evaporation temperature. As a result,  Pu/
Pu/ Pu as well as
Pu as well as  Pu/
Pu/ Pu and
Pu and 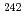 Pu/
Pu/ Pu in plutonium samples could be measured by TIMS with a continuous heating technique and without any chemical separation processes.
Pu in plutonium samples could be measured by TIMS with a continuous heating technique and without any chemical separation processes.
Asai, Shiho; Limbeck, A.*
Talanta, 135, p.41 - 49, 2015/04
Times Cited Count:21 Percentile:58.68(Chemistry, Analytical)A new analytical method for chondrite-normalized Rare earth elements (REE) plot has been developed in this study. Cation-exchange resin particles were used as a substrate to retain REE for laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). In LA analysis, formation of oxide and hydroxide of the light REE and Ba which causes spectral interferences in the heavy REE measurement was effectively attenuated, compared to conventional solution nebulization ICP-MS. To evaluate the applicability of the proposed method, the REE-adsorbed particles prepared by immersing them in a U-bearing solution were measured with LA-ICP-MS. Additionally, each concentration of REE in the U standard solution was determined with solution nebulization ICP-MS. The chondrite-normalized plot obtained through LA-ICP-MS analysis exhibited close agreement with that obtained through the solution nebulization ICP-MS of the REE-separated solution within the uncertainties.
Esaka, Fumitaka; Magara, Masaaki
Talanta, 120, p.349 - 354, 2014/03
Times Cited Count:13 Percentile:40.89(Chemistry, Analytical)Secondary ion mass spectrometry (SIMS) was used in combination with alpha track detection for the efficient analysis of uranium-bearing particles with higher  U abundances in environmental samples. A polycarbonate film containing particles was prepared and placed in contact with a CR-39 plastic detector sheet. After exposure, the sheet was etched in NaOH solution and each uranium-bearing particle was identified through observation of the alpha tracks recorded in the sheet. A portion of the film containing each uranium-bearing particle was cut out and put onto a glassy carbon planchet. The films on the planchet were decomposed through plasma ashing for subsequent uranium abundance ratio analysis with SIMS. The alpha track - SIMS analysis for ten uranium-bearing particles in a sample taken from a nuclear facility afforded
U abundances in environmental samples. A polycarbonate film containing particles was prepared and placed in contact with a CR-39 plastic detector sheet. After exposure, the sheet was etched in NaOH solution and each uranium-bearing particle was identified through observation of the alpha tracks recorded in the sheet. A portion of the film containing each uranium-bearing particle was cut out and put onto a glassy carbon planchet. The films on the planchet were decomposed through plasma ashing for subsequent uranium abundance ratio analysis with SIMS. The alpha track - SIMS analysis for ten uranium-bearing particles in a sample taken from a nuclear facility afforded  (
( U)/
U)/ (
( U) abundance ratios in the range 0.0072 to 0.25, which were significantly higher than those obtained by SIMS without alpha track detection.
U) abundance ratios in the range 0.0072 to 0.25, which were significantly higher than those obtained by SIMS without alpha track detection.
 -diketone and methylphenylphenanthroline carboxamide
-diketone and methylphenylphenanthroline carboxamideHasegawa, Yuko*; Tamaki, Sayaka*; Yajima, Hirofumi*; Hashimoto, Bunji*; Yaita, Tsuyoshi
Talanta, 85(3), p.1543 - 1548, 2011/09
Times Cited Count:38 Percentile:75.86(Chemistry, Analytical)Lee, C. G.; Suzuki, Daisuke; Esaka, Fumitaka; Magara, Masaaki; Kimura, Takaumi
Talanta, 85(1), p.644 - 649, 2011/07
Times Cited Count:16 Percentile:43.45(Chemistry, Analytical)In this study, we developed a method for detecting plutonium particles in a sample mixture of plutonium and uranium particles using alpha track and fission track techniques. The specific radioactivity (Bq/g) for alpha decay of plutonium is several orders of magnitude higher than that of uranium, indicating that the formation of the alpha track due to alpha decay of uranium can be disregarded under suitable conditions. While alpha tracks in addition to fission tracks were detected in a plutonium particle, only fission tracks were detected in a uranium particle, thereby making the alpha tracks an indicator for detecting particles containing plutonium. Using this correlation, the accuracy in isotope ratios, signal intensity and measurement errors are presumable from the number of alpha tracks prior to the isotope ratio measurements by thermal ionization mass spectrometry. It is expected that this method will become an effective tool for plutonium particle analysis.
Esaka, Fumitaka; Magara, Masaaki; Suzuki, Daisuke; Miyamoto, Yutaka; Lee, C. G.; Kimura, Takaumi
Talanta, 83(2), p.569 - 573, 2010/12
Times Cited Count:18 Percentile:46.12(Chemistry, Analytical)In the present work, an analytical technique by a combination of chemical separation and inductively coupled plasma mass spectrometry (ICP-MS) is developed and applied to isotope ratio analysis of individual sub-micrometer plutonium particles. The ICP-MS results for individual plutonium particles prepared from a standard reference material (NIST SRM-947) indicate that the use of a desolvation system for sample introduction improves the precision of isotope ratios. In addition, the accuracy of the  Pu/
Pu/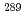 Pu isotope ratio is much improved, owing to the chemical separation of plutonium and americium. In conclusion, the performance of the proposed ICP-MS technique is sufficient for the analysis of individual plutonium particles.
Pu isotope ratio is much improved, owing to the chemical separation of plutonium and americium. In conclusion, the performance of the proposed ICP-MS technique is sufficient for the analysis of individual plutonium particles.
Esaka, Fumitaka; Magara, Masaaki; Lee, C. G.; Sakurai, Satoshi; Usuda, Shigekazu; Shinohara, Nobuo
Talanta, 78(1), p.290 - 294, 2009/04
Times Cited Count:35 Percentile:71.12(Chemistry, Analytical)The determination of uranium isotope ratios in individual particles is of great importance for nuclear safeguards. In the present study, an analytical technique by inductively coupled plasma mass spectrometry (ICP-MS) with a desolvation sample introduction system was applied to isotope ratio analysis of individual uranium particles. In ICP-MS analysis of individual uranium particles with diameters ranging from 0.6 to 4.2  m in a standard reference material (NBL CRM U050), the use of the desolvation system for sample introduction improved the precision of
m in a standard reference material (NBL CRM U050), the use of the desolvation system for sample introduction improved the precision of  U/
U/ U and
U and 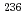 U/
U/ U isotope ratios. The performance of ICP-MS with desolvation was compared with that of a conventionally used method, i.e., secondary ion mass spectrometry (SIMS). The analysis of test swipe samples taken at nuclear facilities implied that the performance of ICP-MS with desolvation was superior to that of SIMS in a viewpoint of accuracy, because the problems of agglomeration of uranium particles and molecular ion interferences by other elements could be avoided. These results indicated that ICP-MS with desolvation has an enough ability to become an effective tool for nuclear safeguards.
U isotope ratios. The performance of ICP-MS with desolvation was compared with that of a conventionally used method, i.e., secondary ion mass spectrometry (SIMS). The analysis of test swipe samples taken at nuclear facilities implied that the performance of ICP-MS with desolvation was superior to that of SIMS in a viewpoint of accuracy, because the problems of agglomeration of uranium particles and molecular ion interferences by other elements could be avoided. These results indicated that ICP-MS with desolvation has an enough ability to become an effective tool for nuclear safeguards.
Esaka, Fumitaka; Esaka, Konomi; Lee, C. G.; Magara, Masaaki; Sakurai, Satoshi; Usuda, Shigekazu; Watanabe, Kazuo
Talanta, 71(3), p.1011 - 1015, 2007/02
Times Cited Count:66 Percentile:87.15(Chemistry, Analytical)A new technique to measure  U/
U/ U and
U and 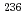 U/
U/ U isotope ratios for individual particles in safeguards environmental samples was developed, which was a combination of particle isolation under scanning electron microscope (SEM) and secondary ion mass spectrometry (SIMS). In order to verify the effectiveness of the technique, the
U isotope ratios for individual particles in safeguards environmental samples was developed, which was a combination of particle isolation under scanning electron microscope (SEM) and secondary ion mass spectrometry (SIMS). In order to verify the effectiveness of the technique, the  U/
U/ U and
U and 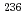 U/
U/ U isotope ratios were measured for individual particles in a simulated environmental sample containing uranium standard (NIST CRM U010) and Pb metal particles. The deviations of the
U isotope ratios were measured for individual particles in a simulated environmental sample containing uranium standard (NIST CRM U010) and Pb metal particles. The deviations of the  U/
U/ U and
U and  U/
U/ U isotope ratios from the certified values increased with increasing the signal intensity ratio of
U isotope ratios from the certified values increased with increasing the signal intensity ratio of  Pb to
Pb to  U, which was due to the interferences by Pb molecular ions. By the isolation of individual uranium particles prior to SIMS analysis, the interferences were eliminated. The effectiveness of the technique was also confirmed by the analysis of a real sample taken at a laboratory in Japan Atomic Energy Agency (JAEA).
U, which was due to the interferences by Pb molecular ions. By the isolation of individual uranium particles prior to SIMS analysis, the interferences were eliminated. The effectiveness of the technique was also confirmed by the analysis of a real sample taken at a laboratory in Japan Atomic Energy Agency (JAEA).
Amano, Hikaru; Yanase, Nobuyuki
Talanta, 37(6), p.585 - 590, 1990/00
Times Cited Count:33 Percentile:83.38(Chemistry, Analytical)no abstracts in English
; ;
Talanta, 34(6), p.567 - 570, 1987/06
Times Cited Count:21 Percentile:73.05(Chemistry, Analytical)no abstracts in English
Talanta, 31(4), p.311 - 314, 1984/00
Times Cited Count:5 Percentile:30.16(Chemistry, Analytical)no abstracts in English
;
Talanta, 31(10A), p.789 - 797, 1984/00
no abstracts in English
Talanta, 26(3), p.251 - 253, 1979/00
Times Cited Count:9no abstracts in English
Talanta, 24(8), p.503 - 507, 1977/08
Times Cited Count:18no abstracts in English
; ;
Talanta, 19, p.329 - 340, 1972/00
Times Cited Count:14no abstracts in English
;
Talanta, 19, p.473 - 478, 1972/00
Times Cited Count:87no abstracts in English