Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Soma, Yasutaka; Komatsu, Atsushi; Igarashi, Takahiro
Dai-71-Kai Zairyo To Kankyo Toronkai Koenshu (CD-ROM), p.253 - 256, 2024/11
In our previous study, we reported that Cl ions penetrating stainless steel crevices do not dissipate by diffusion, even in high-purity water (i.e., conductivity remains stable), likely due to electrochemical reactions inside and outside the crevice. This study further analyzes ion behavior by experimentally and computationally investigating ion concentration drivers in high-purity water. Results show that, at 50 C, the crevice conductivity of SUS316L stainless steel reached 100
C, the crevice conductivity of SUS316L stainless steel reached 100  S/cm (100-1000 times bulk water). Modeling suggests this is due to metal cations and hydroxide ions from dissolved oxygen reduction. The dissolution rate was estimated at 10nA/cm
S/cm (100-1000 times bulk water). Modeling suggests this is due to metal cations and hydroxide ions from dissolved oxygen reduction. The dissolution rate was estimated at 10nA/cm .
.
Takahashi, Rieko*; Taniguchi, Naoki
Zairyo To Kankyo, 73(6), p.153 - 163, 2024/06
Carbon steel is one of the candidate materials for overpacks in geological disposal of high-level radioactive waste, and is known to susceptible to stress corrosion cracking(SCC) depending on the condition in carbonate environment. In order to understand the influence of temperature on the SCC susceptibility of carbon steel, slow strain rate test (SSRT) of rolled steel were performed in NaHCO aqueous solution with varying temperature in the range of 303-393K for conditions of 0.1-0.5 mol/dm
aqueous solution with varying temperature in the range of 303-393K for conditions of 0.1-0.5 mol/dm , which is assumed to be the upper limit of carbonate concentration in groundwater in a geological disposal environment. As the results, no obvious influence of temperature on mechanical properties such as fracture strain ratio and reduction area ratio were observed, but SCC susceptibility based on SCC fracture ratio increased at relatively low temperatures of 303K and 323K. It was suggested that the reason for the higher SCC sensitivity at lower temperatures was due to slower repassivation at lower temperatures. Regarding the type of SCC, intergranular SCC was dominant at low temperatures and tended to transition to intergranular SCC at higher temperatures. Transgranular SCC tended to be observed at lower potentials than those at which intergranular SCC was observed.
, which is assumed to be the upper limit of carbonate concentration in groundwater in a geological disposal environment. As the results, no obvious influence of temperature on mechanical properties such as fracture strain ratio and reduction area ratio were observed, but SCC susceptibility based on SCC fracture ratio increased at relatively low temperatures of 303K and 323K. It was suggested that the reason for the higher SCC sensitivity at lower temperatures was due to slower repassivation at lower temperatures. Regarding the type of SCC, intergranular SCC was dominant at low temperatures and tended to transition to intergranular SCC at higher temperatures. Transgranular SCC tended to be observed at lower potentials than those at which intergranular SCC was observed.
Sato, Tomonori; Hata, Kuniki; Kato, Chiaki; Igarashi, Takahiro
Zairyo To Kankyo, 73(4), p.102 - 109, 2024/04
To evaluate the effects of dissolved oxygen concentration to water quality within SCC crack and the distribution of water quality in the depth direction under irradiation, immersion tests of stainless steel specimens given a gap and water radiolysis calculations for the water quality in the crevice gap were performed. As a result, it was confirmed that Fe O
O was formed in the entire area within the crevice regardless of the dissolved oxygen concentration. It was also estimated that under irradiation, the oxidant species produced directly by radiolysis in the crack are consumed by the oxide growth, and anion enrichment occurs in the crack even in the irradiation conditions.
was formed in the entire area within the crevice regardless of the dissolved oxygen concentration. It was also estimated that under irradiation, the oxidant species produced directly by radiolysis in the crack are consumed by the oxide growth, and anion enrichment occurs in the crack even in the irradiation conditions.
Aoki, So; Sakai, Junichi*
Zairyo To Kankyo, 73(3), p.49 - 56, 2024/03
This paper briefly reviews the history of the development of duplex stainless steels and summarises the grades that are currently standardised in JIS and used industrially. The chemical composition, phase equilibrium and phase transformation of duplex stainless steels are described, showing the basic data. The physical and mechanical properties of the duplex stainless steels are touched upon, and in particular the resistance to uniform corrosion and localised corrosion such as pitting corrosion, crevice corrosion and stress corrosion cracking are outlined, citing previously published data. Finally, the main environments in which duplex stainless steels are used and the results of their use are mentioned.
 Sr and
Sr and  Cs in HCl solutions on the corrosion potential of Type 316L stainless steel
Cs in HCl solutions on the corrosion potential of Type 316L stainless steelAoyama, Takahito; Sato, Tomonori; Ueno, Fumiyoshi; Kato, Chiaki; Sano, Naruto; Yamashita, Naoki; Igarashi, Takahiro
Zairyo To Kankyo, 72(11), p.284 - 288, 2023/11
no abstracts in English
Soma, Yasutaka; Igarashi, Takahiro
Dai-70-Kai Zairyo To Kankyo Toronkai Koenshu (CD-ROM), p.199 - 202, 2023/10
Since an acidic corrosive environment (crevice environment) is formed inside the stress corrosion cracking (SCC) of stainless steel in high temperature water, it is important to understand the corrosion behavior in the crevice environment for the better understanding of crack growth behavior. In the previous study, the authors measured the electrical conductivity inside the crevice and obtained values of 380  S/cm and 1600
S/cm and 1600  S/cm for the crevice with and without intergranular corrosion, respectively. In this study, we defined the crevice environment I (pH
S/cm for the crevice with and without intergranular corrosion, respectively. In this study, we defined the crevice environment I (pH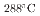 =4.41) and II (pH
=4.41) and II (pH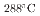 =3.13) corresponding the above conductivity values, and the corrosion behavior of Fe-xCr-20Ni (x=16.9, 19.8, 22.9, 24.3, 25.9) in each crevice environment was investigated. In the simulated crevice environment-I, the all alloys showed passive behavior, while in the environment-II, severe corrosion with intergranular cracking was observed for x = 16.9 and 19.8, and a thick oxide film was formed. On the other hand, above x=22.9, oxide film growth was suppressed and a clear passive region appeared on the polarization curve.
=3.13) corresponding the above conductivity values, and the corrosion behavior of Fe-xCr-20Ni (x=16.9, 19.8, 22.9, 24.3, 25.9) in each crevice environment was investigated. In the simulated crevice environment-I, the all alloys showed passive behavior, while in the environment-II, severe corrosion with intergranular cracking was observed for x = 16.9 and 19.8, and a thick oxide film was formed. On the other hand, above x=22.9, oxide film growth was suppressed and a clear passive region appeared on the polarization curve.
Hata, Kuniki; Kimura, Atsushi*; Taguchi, Mitsumasa*; Sato, Tomonori; Kato, Chiaki; Watanabe, Yutaka*
Zairyo To Kankyo, 72(4), p.126 - 130, 2023/04
Gamma-radiolysis experiments with gas-liquid coexistent samples were carried out to investigate effects of gas-phase radiolysis on corrosive environment for materials in solutions under irradiation. After gamma-ray irradiation, hydrogen peroxide, nitrate ion, nitrite ion were detected in the liquid phase. The production yields of nitrate ion and nitrite ion increased with increasing gas-phase volume and oxygen concentration. This result indicated that chemical reactions including oxygen and nitrogen in the gas phase were required for the production of nitrate ion and nitrite ion. To magnify the effects of gas-phase radiolysis in the gas-liquid coexistent samples, absorption dose rate in the liquid phase was reduced by one-hundredth using lead shield. The concentration of hydrogen peroxide and the pH in the shielded liquid phase were similar to those in the irradiated pure water, which did not contact with gas phase. This result indicated that the effects of nitrate ion and nitrite ion dissolved in the liquid phase on water radiolysis were not important in the current experimental system, in which the effects of gas-phase radiolysis were increased by 100-times.
Momma, Yuichiro*; Sakairi, Masatoshi*; Ueno, Fumiyoshi; Otani, Kyohei
Zairyo To Kankyo, 71(5), p.133 - 137, 2022/05
The effect of the corrosion inhibitor on the corrosion of steel under a thin solution layer was investigated. As a result of forming a thin solution layer with a thickness of 1.0-0.2 mm on the specimen, adding a mixed solution of sodium molybdate and aluminum lactate as a corrosion inhibitor, and performing electrochemical measurement, the corrosion inhibitor suppresses the anodic reaction. And in the thin solution layer, it was suggested that the morphology of the protective layer structure by the corrosion inhibitor changed according to the amount of liquid as compared with the bulk immersion.
Soma, Yasutaka; Kato, Chiaki
Zairyo To Kankyo 2022 Koenshu (CD-ROM), p.219 - 220, 2022/05
It is important to understand the electrochemical properties of stainless steel in environment created within crevice of stainless steel in high temperature water (crevice environment). This is because acidification and concentration of impurity ions occur in the crevice environment and this is common inside the stress corrosion crack. In this study, we reproduced the crevice environment in bulk scale and investigated mainly the effect of Cr concentration on the electrochemical properties of Fe-Cr-Ni alloys. Polarization curves of Fe-20Ni-xCr (x=16.4, 23, 26) were measured in water with a temperature of 288 C, a Cl concentration of 2
C, a Cl concentration of 2 10
10 mol/dm
mol/dm , a pH value of about 4.5, and a dissolved hydrogen concentration of 10 ppb. The peak currents of active dissolution (at -400 mV) and passive current density (at -50 mV) for specimens with Cr concentrations x = 16.4, 23, and 26% were approximately 13.8, 15.9, 10.0
, a pH value of about 4.5, and a dissolved hydrogen concentration of 10 ppb. The peak currents of active dissolution (at -400 mV) and passive current density (at -50 mV) for specimens with Cr concentrations x = 16.4, 23, and 26% were approximately 13.8, 15.9, 10.0  Acm
Acm , and 18.4, 8.5, 8.5
, and 18.4, 8.5, 8.5  Acm
Acm , respectively. Although the current values of x=26 were slightly lower in both cases, it was concluded that there was no clear dependence of the polarization curve on Cr concentration in this environment.
, respectively. Although the current values of x=26 were slightly lower in both cases, it was concluded that there was no clear dependence of the polarization curve on Cr concentration in this environment.
 Sr dissolved solution on corrosion potential of type 316L stainless steel
Sr dissolved solution on corrosion potential of type 316L stainless steelAoyama, Takahito; Kato, Chiaki; Sato, Tomonori; Sano, Naruto; Yamashita, Naoki; Ueno, Fumiyoshi
Zairyo To Kankyo, 71(4), p.110 - 115, 2022/04
no abstracts in English
Momma, Yuichiro*; Sakairi, Masatoshi*; Ueno, Fumiyoshi; Otani, Kyohei
Zairyo To Kankyo, 71(4), p.121 - 125, 2022/04
The effect of solution layer thickness on the atmospheric corrosion of carbon steel was investigated using novel devices fabricated by a 3D printer. These novel devices allowed us to control the solution layer thickness precisely. Potentiodynamic polarization measurements were performed under thickness-controlled solution layer, and oxygen diffusion limiting current density ( ) and anodic current density (
) and anodic current density ( ) were measured. As the solution layer become thinner,
) were measured. As the solution layer become thinner,  increased and
increased and  decreased. This result indicates that corrosion accelerates when the solution layer becomes thinner. The diffusion coefficient of oxygen was calculated as 3.20
decreased. This result indicates that corrosion accelerates when the solution layer becomes thinner. The diffusion coefficient of oxygen was calculated as 3.20 10
10 cm
cm s
s from the relationship between
from the relationship between  and solution layer thickness, and the critical diffusion thickness was estimated to be 0.87 mm.
and solution layer thickness, and the critical diffusion thickness was estimated to be 0.87 mm.
 Np
NpIrisawa, Eriko; Kato, Chiaki; Yamashita, Naoki; Sano, Naruto
Zairyo To Kankyo, 71(3), p.70 - 74, 2022/03
In order to evaluate the corrosion of stainless steels used in spent nuclear fuel reprocessing facilities, the immersion corrosion tests and polarization measurements were performed using R-SUS304ULC stainless steel in nitric acid solution containing a kind of radionuclides,  Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of
Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of 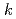 =0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
=0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
Otani, Kyohei; Ueno, Fumiyoshi; Kato, Chiaki
Zairyo To Kankyo, 71(2), p.40 - 45, 2022/02
The purpose of this study is to investigate the effect of oxygen concentration in the air on the corrosion rate of carbon steel in an air/solution alternating environment in the low oxygen concentration range and to clarify the corrosion rate and corrosion mechanism of carbon steel depending on the oxygen concentration in air by the mass change of specimens before and after the corrosion test and observing the iron rust layer formed on the surface of carbon steel. The corrosion rate increases with increasing oxygen concentration in the air, and the gradient of the corrosion rate decreases gradually. The maximum erosion depth increased with increasing oxygen concentration except for the case of 1% oxygen concentration, however, the maximum erosion depth for 1% oxygen concentration was larger than that for 5% air oxygen concentration.
Kato, Chiaki; Yamagishi, Isao; Sato, Tomonori; Yamamoto, Masahiro*
Zairyo To Kankyo, 70(12), p.441 - 447, 2021/12
Zeolite particles have been used in a Cs adsorption vessel for purification of contaminated water in Fukushima Daiichi Nuclear Power Station (1F). The used Cs adsorption vessels were kept in storage space on 1F site. The risk of localized corrosion of stainless steel used in the vessel was worried. To evaluate the risk of localized corrosion, using specially designed electrochemical testing apparatus was used under gamma-ray irradiation test. And, real size mock-up test conducted. The results showed the potential change caused by creation of H O
O by water radiolysis decreased by zeolite particles and the enrichment of chloride ion concentration in the vessel do not propagate during dry up procedure of Cs adsorption vessel. These data indicate the risk of localized corrosion of Cs adsorption vessel may stay at considerably low level.
by water radiolysis decreased by zeolite particles and the enrichment of chloride ion concentration in the vessel do not propagate during dry up procedure of Cs adsorption vessel. These data indicate the risk of localized corrosion of Cs adsorption vessel may stay at considerably low level.
Wakai, Satoshi*; Hirano, Shinichi*; Ueno, Fumiyoshi; Okamoto, Akihiro*
Zairyo To Kankyo, 70(12), p.491 - 496, 2021/12
After Fukushima Daiichi Nuclear Power Station accident, various corrosion mitigating activities have been treated, and severe corrosion incident have never taken placed. On the other hand, the facilities were exposed sea water, and some of them have continuously exposed to ground water. The exposure of metal materials to environmental water has a risk of microbiologically influenced corrosion (MIC). In this paper, we summarize the latest knowledge of MIC and the task of MIC in the decommissioning of Fukushima Daiichi Nuclear Power Station.
Otani, Kyohei; Kato, Chiaki
Zairyo To Kankyo, 70(12), p.480 - 486, 2021/12
This is a comprehensive paper of the corrosion of carbon steel in air/solution alternating condition. From cross-sectional observation and analysis of the iron rust layer formed on the surface of carbon steel in the alternating condition, it was found that a multilayered iron rust layer composed of red rust layer ( -FeOOH), rust crust layer (Fe
-FeOOH), rust crust layer (Fe O
O ), inner crystal (Fe
), inner crystal (Fe O
O ), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
Hata, Kuniki
Zairyo To Kankyo, 70(12), p.468 - 473, 2021/12
In order to estimate corrosive environment in the contaminated water at Fukushima Daiichi Nuclear Power Station, effects of oxidants, such as H O
O , which were generated from water radiolysis, should be taken into account due to the irradiation field in the reactor building. The process of water radiolysis and the amounts of these oxidants can change depending on the conditions of water and types of radiation. After the accident, a variety of factors, which can affect water radiolysis, such as seawater constituents, surface of oxides, and
, which were generated from water radiolysis, should be taken into account due to the irradiation field in the reactor building. The process of water radiolysis and the amounts of these oxidants can change depending on the conditions of water and types of radiation. After the accident, a variety of factors, which can affect water radiolysis, such as seawater constituents, surface of oxides, and  -radionuclides, had been discussed. In this paper, these effects on radiolysis are reviewed for the better understanding of the corrosive environment in the contaminated water.
-radionuclides, had been discussed. In this paper, these effects on radiolysis are reviewed for the better understanding of the corrosive environment in the contaminated water.
Sato, Tomonori; Komatsu, Atsushi; Nakano, Junichi*; Yamamoto, Masahiro*
Zairyo To Kankyo, 70(12), p.457 - 461, 2021/12
The Primary Containment Vessel (PCV) in Fukushima Daiichi Nuclear Power Plants (1F) have been exposed to a corrosive environment containing seawater components under irradiation condition. In such conditions, the hydrogen peroxide (H O
O ) is generated by water radiolysis as an oxidant in addition to dissolved oxygen. The H
) is generated by water radiolysis as an oxidant in addition to dissolved oxygen. The H O
O concentration is able to be estimated by radiolysis calculations. The corrosion tests under gamma-ray irradiation was performed. As the results, it was confirmed that the N
concentration is able to be estimated by radiolysis calculations. The corrosion tests under gamma-ray irradiation was performed. As the results, it was confirmed that the N gas purging performed in the PCV is effective in inhibiting the corrosion of carbon steel under irradiation conditions. The weight loss of carbon steel obtained in the corrosion test under gamma-ray irradiations, are evaluated using calculated O
gas purging performed in the PCV is effective in inhibiting the corrosion of carbon steel under irradiation conditions. The weight loss of carbon steel obtained in the corrosion test under gamma-ray irradiations, are evaluated using calculated O and H
and H O
O concentrations obtained by radiolysis calculations. As a results, it is confirmed that the corrosion rate of carbon steel under irradiation is determined by the sum of diffusion limiting currents of O
concentrations obtained by radiolysis calculations. As a results, it is confirmed that the corrosion rate of carbon steel under irradiation is determined by the sum of diffusion limiting currents of O and H
and H O
O as well as that of carbon steel in the O
as well as that of carbon steel in the O and H
and H O
O coexistent conditions under non-irradiation.
coexistent conditions under non-irradiation.
Soma, Yasutaka; Kato, Chiaki
Dai-68-Kai Zairyo To Kankyo Toronkai Koenshu (CD-ROM), p.205 - 206, 2021/10
This study investigates the effect of temperature on dissipation behavior of Cl ion within the crevice of stainless steel. Concentration of Cl ion was evaluated by conductivity measured by using sensors installed at crevice specimen. At 50 and 80  C, Cl ions within the crevice of PEEK and Pt dissipated in accordance with concentration diffusion. On the contrary, dissipation speed of Cl ions inside the Type-304L stainless steel were much lower than those anticipated by simple concentration diffusion. This behavior attribute to the anodic dissolution of stainless steel inside the crevice, therefore, to quantitatively understand the effect of temperature on the dissipation behavior, it is necessary to know the anodic dissolution rate and occurrence of localized corrosion. Numerical analysis taking the effect of concentration diffusion and migration into account is also needed.
C, Cl ions within the crevice of PEEK and Pt dissipated in accordance with concentration diffusion. On the contrary, dissipation speed of Cl ions inside the Type-304L stainless steel were much lower than those anticipated by simple concentration diffusion. This behavior attribute to the anodic dissolution of stainless steel inside the crevice, therefore, to quantitatively understand the effect of temperature on the dissipation behavior, it is necessary to know the anodic dissolution rate and occurrence of localized corrosion. Numerical analysis taking the effect of concentration diffusion and migration into account is also needed.
Ishijima, Yasuhiro; Ueno, Fumiyoshi; Abe, Hitoshi
Zairyo To Kankyo, 70(6), p.192 - 198, 2021/06
The time dependence of corrosion behavior on tantalum used in nuclear fuel reprocessing equipment in sodium hydroxide solution was investigated by immersion corrosion tests, and the mechanism of aging change was discussed from surface observations and electrochemical measurements. The immersion tests were carried out at room temperature with NaOH concentrations ranging from 1 to 7 mol/L and immersion times ranging from 24 to 168 hr, respectively. The corrosion rate increased with NaOH concentration, but peaked with immersion time and then decreased. The time to peak of corrosion rate was shorter with higher NaOH concentration. The SEM observations and Raman analysis at the surface of the specimens that were cleaned and weighed after the immersion test did not show any film formation. On the other hand, the polarization resistance showed a constant value or an increase after a decrease immediately after immersion. It is suggested that the change in corrosion rate is affected by the formation of film by immersion, since the value of polarization resistance is almost the same as the sum of film resistance and charge transfer resistance. The film was considered to be mainly Na Ta
Ta O
O formed by the dissolution of Ta.
formed by the dissolution of Ta.