Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Sn in concrete rubble
Sn in concrete rubbleDo, V. K.; Furuse, Takahiro; Ota, Yuki; Iwahashi, Hiroyuki; Hirosawa, Takashi; Watanabe, Masahisa; Sato, Soichi
Journal of Radioanalytical and Nuclear Chemistry, 331(12), p.5631 - 5640, 2022/12
Times Cited Count:5 Percentile:53.26(Chemistry, Analytical) Sn is one of the long-lived fission products that might have been released into the environment after the Fukushima nuclear accident in Japan in 2011. The presence of radionuclides must be monitored for the proper treatment of wastes obtained from decommissioning accident-related nuclear facilities and the surrounding environment. In the work, we propose a reliable method for verifying the presence of
Sn is one of the long-lived fission products that might have been released into the environment after the Fukushima nuclear accident in Japan in 2011. The presence of radionuclides must be monitored for the proper treatment of wastes obtained from decommissioning accident-related nuclear facilities and the surrounding environment. In the work, we propose a reliable method for verifying the presence of  Sn in construction materials by combining the HCl-free solid phase extraction on TEVA resin and a selective measurement by inductively coupled plasma tandem mass spectrometry (ICP-MS/MS). The method has been optimized and characterized step by step. More than 95% of chemical recovery was achieved for Sn from typical concrete matrixes. The interference caused by an isobar
Sn in construction materials by combining the HCl-free solid phase extraction on TEVA resin and a selective measurement by inductively coupled plasma tandem mass spectrometry (ICP-MS/MS). The method has been optimized and characterized step by step. More than 95% of chemical recovery was achieved for Sn from typical concrete matrixes. The interference caused by an isobar  Te and possible polyatomic interferences from matrixes were effectively suppressed by the developed chemical separation and the tandem MS/MS configuration. The total decontamination factor for the Te interference was of the order of 10
Te and possible polyatomic interferences from matrixes were effectively suppressed by the developed chemical separation and the tandem MS/MS configuration. The total decontamination factor for the Te interference was of the order of 10 . The estimated method detection limit for
. The estimated method detection limit for  Sn in concrete as measured at m/z = 160 was 12.1 pg g
Sn in concrete as measured at m/z = 160 was 12.1 pg g , which is equivalent to 6.1 mBq g
, which is equivalent to 6.1 mBq g .
.
Oda, Chie;
JNC TN8400 98-001, 14 Pages, 1998/11
Solubilities of amorphous stannic oxide, SnO (am) in Na-ClO
(am) in Na-ClO -Cl-SO
-Cl-SO aqueous systems were measured to quantitatively investigate the influences of the ligands OH
aqueous systems were measured to quantitatively investigate the influences of the ligands OH , Cl
, Cl and SO
and SO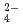 on solubilities. They were also measured in bentonite equilibrated solutions to discuss the behavior of tin under a repository condition of a high-revel radioactive waste. The solubility data in sodium perchlorate media in the range of pH from 6 to 11 showed pH dependency, and the hydrolysis constants of tin (IV) were determined (Amaya, et al., 1997). No significant changes in solubilities with the variation in Cl
on solubilities. They were also measured in bentonite equilibrated solutions to discuss the behavior of tin under a repository condition of a high-revel radioactive waste. The solubility data in sodium perchlorate media in the range of pH from 6 to 11 showed pH dependency, and the hydrolysis constants of tin (IV) were determined (Amaya, et al., 1997). No significant changes in solubilities with the variation in Cl , SO
, SO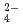 concentrations were observed in Na-ClO
concentrations were observed in Na-ClO -Cl-SO
-Cl-SO aqueous systems, so this indicates that chloride and sulfate species were less effective than hydroxide complexes. On the other hand, solubilities in bentonite equilibrated solutions were higher than solubilities of other experiments in simple systems. These results suggest that (1) other complexes of tin except hydroxide, chloride or sulfate complexes of tin (IV) may dominantly exist in aqueous phase, (2)solid phase other than SnO
aqueous systems, so this indicates that chloride and sulfate species were less effective than hydroxide complexes. On the other hand, solubilities in bentonite equilibrated solutions were higher than solubilities of other experiments in simple systems. These results suggest that (1) other complexes of tin except hydroxide, chloride or sulfate complexes of tin (IV) may dominantly exist in aqueous phase, (2)solid phase other than SnO (am) may limit the solubility of tin under repository conditions.
(am) may limit the solubility of tin under repository conditions.
 Sn in radioactive rubbles by ICP-MS/MS
Sn in radioactive rubbles by ICP-MS/MSOta, Yuki; Do, V. K.; Furuse, Takahiro; Sano, Yuichi; Iwahashi, Hiroyuki; Homma, Shunta; Ichijo, Yurina; Kurosawa, Kiyoko*; Endo, Tsubasa*; Motoki, Yoshiaki*; et al.
no journal, ,
no abstracts in English