Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Soma, Yasutaka; Komatsu, Atsushi; Kaji, Yoshiyuki; Yamamoto, Masahiro*; Igarashi, Takahiro
Corrosion Science, 251, p.112897_1 - 112897_15, 2025/07
Times Cited Count:1 Percentile:61.86(Materials Science, Multidisciplinary)Experimental and modeling studies of the oxygen ingression at the crevices of stainless steels were conducted in high-temperature water (288 C). The limiting distance of oxygen ingression,
C). The limiting distance of oxygen ingression, 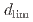 , was defined as the point beyond which the primary surface oxide changed (hematite
, was defined as the point beyond which the primary surface oxide changed (hematite  magnetite), regardless of crevice gap, oxygen concentration, and time. In situ measurements revealed increased electrical conductivity around the
magnetite), regardless of crevice gap, oxygen concentration, and time. In situ measurements revealed increased electrical conductivity around the 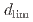 position indicating ion enrichment due to a differential oxygen concentration cell.
position indicating ion enrichment due to a differential oxygen concentration cell. 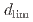 increased with increasing crevice gap, oxygen concentration, and immersion time. Modeling study suggested that oxide layer growth reduced anodic dissolution and slowed oxygen consumption, allowing oxygen ingression with time.
increased with increasing crevice gap, oxygen concentration, and immersion time. Modeling study suggested that oxide layer growth reduced anodic dissolution and slowed oxygen consumption, allowing oxygen ingression with time.
Soma, Yasutaka; Komatsu, Atsushi; Igarashi, Takahiro
Dai-71-Kai Zairyo To Kankyo Toronkai Koenshu (CD-ROM), p.253 - 256, 2024/11
In our previous study, we reported that Cl ions penetrating stainless steel crevices do not dissipate by diffusion, even in high-purity water (i.e., conductivity remains stable), likely due to electrochemical reactions inside and outside the crevice. This study further analyzes ion behavior by experimentally and computationally investigating ion concentration drivers in high-purity water. Results show that, at 50 C, the crevice conductivity of SUS316L stainless steel reached 100
C, the crevice conductivity of SUS316L stainless steel reached 100  S/cm (100-1000 times bulk water). Modeling suggests this is due to metal cations and hydroxide ions from dissolved oxygen reduction. The dissolution rate was estimated at 10nA/cm
S/cm (100-1000 times bulk water). Modeling suggests this is due to metal cations and hydroxide ions from dissolved oxygen reduction. The dissolution rate was estimated at 10nA/cm .
.
Soma, Yasutaka; Komatsu, Atsushi; Ueno, Fumiyoshi
Corrosion, 78(6), p.503 - 515, 2022/06
Times Cited Count:1 Percentile:5.82(Materials Science, Multidisciplinary)The effects of electrochemical potential (ECP) on water chemistry within a crevice are of critical importance for understanding stress corrosion cracking (SCC) of Fe-Cr-Ni alloys in high temperature water. In this study, the effects of ECP on the electrical conductivity of a solution within a Type-316L stainless steel crevice (
 ) have been studied in 288
) have been studied in 288 C and 8 MPa water containing 10 ppb Cl
C and 8 MPa water containing 10 ppb Cl as major anionic species. In situ measurements of
as major anionic species. In situ measurements of 
 in a rectangular crevice with a gap of 15
in a rectangular crevice with a gap of 15  m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in
m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in 
 from 12
from 12  Scm
Scm to 160
to 160  Scm
Scm at a distance of 21 mm from the crevice mouth. The increase in
at a distance of 21 mm from the crevice mouth. The increase in 
 reached a maximum at about 0.15 V (about 300
reached a maximum at about 0.15 V (about 300  Scm
Scm ) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl
) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl is the major anionic species transported into the crevice at relatively low potentials, and that
is the major anionic species transported into the crevice at relatively low potentials, and that 
 increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
 generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
Soma, Yasutaka; Kato, Chiaki
Zairyo To Kankyo 2022 Koenshu (CD-ROM), p.219 - 220, 2022/05
It is important to understand the electrochemical properties of stainless steel in environment created within crevice of stainless steel in high temperature water (crevice environment). This is because acidification and concentration of impurity ions occur in the crevice environment and this is common inside the stress corrosion crack. In this study, we reproduced the crevice environment in bulk scale and investigated mainly the effect of Cr concentration on the electrochemical properties of Fe-Cr-Ni alloys. Polarization curves of Fe-20Ni-xCr (x=16.4, 23, 26) were measured in water with a temperature of 288 C, a Cl concentration of 2
C, a Cl concentration of 2 10
10 mol/dm
mol/dm , a pH value of about 4.5, and a dissolved hydrogen concentration of 10 ppb. The peak currents of active dissolution (at -400 mV) and passive current density (at -50 mV) for specimens with Cr concentrations x = 16.4, 23, and 26% were approximately 13.8, 15.9, 10.0
, a pH value of about 4.5, and a dissolved hydrogen concentration of 10 ppb. The peak currents of active dissolution (at -400 mV) and passive current density (at -50 mV) for specimens with Cr concentrations x = 16.4, 23, and 26% were approximately 13.8, 15.9, 10.0  Acm
Acm , and 18.4, 8.5, 8.5
, and 18.4, 8.5, 8.5  Acm
Acm , respectively. Although the current values of x=26 were slightly lower in both cases, it was concluded that there was no clear dependence of the polarization curve on Cr concentration in this environment.
, respectively. Although the current values of x=26 were slightly lower in both cases, it was concluded that there was no clear dependence of the polarization curve on Cr concentration in this environment.
Soma, Yasutaka; Kato, Chiaki
Dai-68-Kai Zairyo To Kankyo Toronkai Koenshu (CD-ROM), p.205 - 206, 2021/10
This study investigates the effect of temperature on dissipation behavior of Cl ion within the crevice of stainless steel. Concentration of Cl ion was evaluated by conductivity measured by using sensors installed at crevice specimen. At 50 and 80  C, Cl ions within the crevice of PEEK and Pt dissipated in accordance with concentration diffusion. On the contrary, dissipation speed of Cl ions inside the Type-304L stainless steel were much lower than those anticipated by simple concentration diffusion. This behavior attribute to the anodic dissolution of stainless steel inside the crevice, therefore, to quantitatively understand the effect of temperature on the dissipation behavior, it is necessary to know the anodic dissolution rate and occurrence of localized corrosion. Numerical analysis taking the effect of concentration diffusion and migration into account is also needed.
C, Cl ions within the crevice of PEEK and Pt dissipated in accordance with concentration diffusion. On the contrary, dissipation speed of Cl ions inside the Type-304L stainless steel were much lower than those anticipated by simple concentration diffusion. This behavior attribute to the anodic dissolution of stainless steel inside the crevice, therefore, to quantitatively understand the effect of temperature on the dissipation behavior, it is necessary to know the anodic dissolution rate and occurrence of localized corrosion. Numerical analysis taking the effect of concentration diffusion and migration into account is also needed.

 generation; The Role of NO
generation; The Role of NO
 in the crevice corrosion repassivation of type 316L stainless steel
in the crevice corrosion repassivation of type 316L stainless steelAoyama, Takahito; Sugawara, Yu*; Muto, Izumi*; Hara, Nobuyoshi*
Journal of the Electrochemical Society, 166(10), p.C250 - C260, 2019/01
Times Cited Count:7 Percentile:18.19(Electrochemistry)The role of NO
 in the repassivation of crevice corrosion of Type 316L stainless steel was investigated. In crevice corrosion tests, the solution was changed from 1 M NaCl to NaCl-NaNO
in the repassivation of crevice corrosion of Type 316L stainless steel was investigated. In crevice corrosion tests, the solution was changed from 1 M NaCl to NaCl-NaNO . NO
. NO
 led to complete repassivation. Repassivation of the crevice corrosion was found to take place in two steps. In the first step, the estimated current density inside the crevice gradually decreased from ca. 5 mA cm
led to complete repassivation. Repassivation of the crevice corrosion was found to take place in two steps. In the first step, the estimated current density inside the crevice gradually decreased from ca. 5 mA cm to ca. 5
to ca. 5  A cm
A cm . After that, the current density suddenly decreased to less than 0.1
. After that, the current density suddenly decreased to less than 0.1  A cm
A cm . From the potentiodynamic polarization in acidic solutions simulated inside the crevice (pH 0.2) and in situ observations of the crevice corrosion morphology, the first step was thought to be generated by the suppression of active dissolution by NO
. From the potentiodynamic polarization in acidic solutions simulated inside the crevice (pH 0.2) and in situ observations of the crevice corrosion morphology, the first step was thought to be generated by the suppression of active dissolution by NO
 . It would appear that the generation of NH
. It would appear that the generation of NH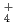 results in a pH increase and the further suppression of active dissolution, and then repassivation occurs.
results in a pH increase and the further suppression of active dissolution, and then repassivation occurs.
Soma, Yasutaka; Komatsu, Atsushi; Ueno, Fumiyoshi
Zairyo To Kankyo, 67(9), p.381 - 385, 2018/09
In-situ measurement of electrical conductivity of solution within crevice of SUS316L stainless steel in 288 C water has been conducted with newly developed electrochemical sensor system. The sensor measures local electrical conductivity of crevice solution beneath the electrode (
C water has been conducted with newly developed electrochemical sensor system. The sensor measures local electrical conductivity of crevice solution beneath the electrode (
 ) with electrochemical impedance method. The sensors were installed at different positions within tapered crevice of SUS316L stainless steel. The crevice specimen with the sensors were immerged into 288
) with electrochemical impedance method. The sensors were installed at different positions within tapered crevice of SUS316L stainless steel. The crevice specimen with the sensors were immerged into 288 C, 8 MPa, pure oxygen saturated high purity water for 100 h.
C, 8 MPa, pure oxygen saturated high purity water for 100 h. 
 at a position with crevice gap of
at a position with crevice gap of  59.3
59.3 m was 8-11
m was 8-11 S/cm, least deviate from conductivity of 288
S/cm, least deviate from conductivity of 288 C pure water (4.4
C pure water (4.4 S/cm) and no localized corrosion occurred. On the contrary,
S/cm) and no localized corrosion occurred. On the contrary, 
 at a position with crevice gap of
at a position with crevice gap of  4.4
4.4 m increased with time and showed maximum value of
m increased with time and showed maximum value of  1600
1600 S/cm at 70 h. Localized corrosion occurred in the vicinity of this position. Thermodynamic equilibrium calculation showed
S/cm at 70 h. Localized corrosion occurred in the vicinity of this position. Thermodynamic equilibrium calculation showed 
 of 1600
of 1600 S/cm being equivalent to pH of 3 to 3.7. It can be concluded that acidification occurred in tight crevice even under high purity bulk water and resulted in localized corrosion.
S/cm being equivalent to pH of 3 to 3.7. It can be concluded that acidification occurred in tight crevice even under high purity bulk water and resulted in localized corrosion.
Soma, Yasutaka; Ueno, Fumiyoshi
Zairyo To Kankyo, 67(5), p.222 - 228, 2018/05
Localized corrosion in crevice of SUS316 stainless steel after immersion in 288 C high purity water with dissolved oxygen concentration of 32 ppm for 100 h was analyzed. Two different types of localized corrosion initiated on grain boundary and inclusions. The former initiated on grain boundary and oxide grown into grain matrix. The oxidized area showed duplex structure composed of microcrystalline FeCr
C high purity water with dissolved oxygen concentration of 32 ppm for 100 h was analyzed. Two different types of localized corrosion initiated on grain boundary and inclusions. The former initiated on grain boundary and oxide grown into grain matrix. The oxidized area showed duplex structure composed of microcrystalline FeCr O
O and island-shaped residual metals. The latter initiated on inclusions containing Ca and S and microcrystalline FeCr
and island-shaped residual metals. The latter initiated on inclusions containing Ca and S and microcrystalline FeCr O
O grown into metal matrix. These localized corrosion occurred selectively in oxygen depleted area indicated formation of macroscopic corrosion cell with the corroded area as anode and surrounding oxygenated area as cathode.
grown into metal matrix. These localized corrosion occurred selectively in oxygen depleted area indicated formation of macroscopic corrosion cell with the corroded area as anode and surrounding oxygenated area as cathode.
Soma, Yasutaka; Kato, Chiaki; Ueno, Fumiyoshi
Proceedings of the 18th International Conference on Environmental Degradation of Materials in Nuclear Power Systems - Water Reactors, Vol.2, p.509 - 521, 2018/00
In-situ electrochemical measurement within crevice of stainless steel in 288 C water has been conducted to analyze crevice water chemistry. Small sensors (
C water has been conducted to analyze crevice water chemistry. Small sensors (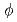
 250
250 m) measured local solution electrical conductivity,
m) measured local solution electrical conductivity, 
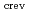 , polarization resistance, and electrochemical corrosion potential. Real-time response of the
, polarization resistance, and electrochemical corrosion potential. Real-time response of the 
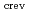 as functions of bulk water conductivity, dissolved oxygen (DO) concentration has been quantitatively analyzed. The effect of geometrical factors on the crevice environment was also studied. The
as functions of bulk water conductivity, dissolved oxygen (DO) concentration has been quantitatively analyzed. The effect of geometrical factors on the crevice environment was also studied. The 
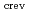 differ more than an order of magnitude depending on the oxygen potential inside the crevice. The
differ more than an order of magnitude depending on the oxygen potential inside the crevice. The 
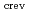 increased by small amount of bulk DO (e.g. 30 ppb). Maximum
increased by small amount of bulk DO (e.g. 30 ppb). Maximum 
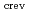 was observed with DO of 32000 ppb and became more than 100 times higher than that of bulk water. Crevice geometry affected significantly on the water chemistry inside.
was observed with DO of 32000 ppb and became more than 100 times higher than that of bulk water. Crevice geometry affected significantly on the water chemistry inside.
; Takizuka, Takakazu
Journal of Nuclear Science and Technology, 22(5), p.387 - 397, 1985/00
Times Cited Count:6 Percentile:63.89(Nuclear Science & Technology)no abstracts in English
; Takizuka, Takakazu;
JAERI-M 84-128, 19 Pages, 1984/07
no abstracts in English
Soma, Yasutaka
no journal, ,
Commemorative speech for "The Award of JSCE for young researcher" of Japan Society of Corrosion Engineering on May 21st 2020, entitled "Characterization of the mechanism of localized corrosion in the crevices of stainless steel in high-temperature, high-purity water" will be made. In this study, we conducted followings: (i) Corrosion test of Type 316L stainless steel to analyze susceptibility to localized corrosion within a crevice in 561K high purity water, and (ii) Develop a sensor system to measure the solution conductivity in a crevice and study relationship between crevice water chemistry and the localized corrosion. These studies were done for the purpose of clarifying the mechanism of stress corrosion cracking (SCC). It was shown that Type-316L stainless steel is susceptible to intergranular corrosion inside the crevice. The developed sensors detected very high solution conductivity in the vicinity of the intergranular corroded area indicate highly corrosive environments were formed in crevice with small gaps. This system can be applied to clarify the mechanism of corrosion related failure, such as SCC, and is expected to contribute to the safety improvement of nuclear reactors.
Soma, Yasutaka; Komatsu, Atsushi; Kato, Chiaki
no journal, ,
In this study, the effect of impurities in steel and ppb level of chloride in bulk water on electrical conductivity of stainless steel's crevice solution (K) has been studied. Crevice specimens were made of as-polished Type-316L stainless steel (standard-SS), standard-SS exposed to 60% nitric acid to dissolve sulfur containing inclusions (acid-picked SS), and 316EHP steel in which sulfur and phosphorous concentration was decreased compared to standard-SS (EHP-SS). These crevice specimens were immersed into 561 K, 8 MPa water the K values were measured as a function of time with stepwise increase in dissolved oxygen levels. In addition, effect of 50 ppb Cl added to bulk water was investigated using standard-SS crevice. The all of the standard-SS, acid-picked SS, and 316EHP showed similar K vs time curves. It can be concluded that impurities dissolved from the steel itself do not significantly contribute to the increase of K. The effect of 50 ppb Cl
added to bulk water was investigated using standard-SS crevice. The all of the standard-SS, acid-picked SS, and 316EHP showed similar K vs time curves. It can be concluded that impurities dissolved from the steel itself do not significantly contribute to the increase of K. The effect of 50 ppb Cl on K vs time curve was obvious because maximum K value became more that 2 times larger than the solution without Cl
on K vs time curve was obvious because maximum K value became more that 2 times larger than the solution without Cl addition. This indicate that small concentration of impurities can be migrated into the crevice.
addition. This indicate that small concentration of impurities can be migrated into the crevice.
Soma, Yasutaka; Komatsu, Atsushi; Ueno, Fumiyoshi; Inagaki, Hiromitsu*
no journal, ,
Corrosion condition within crevice of stainless steel is important to understand dissolution mechanisms of crack tip of stress corrosion cracking (SCC) in high-temperature water. We have reported that electrical conductivity of solution within crevice of stainless steel ( crev) exposed to high temperature and high purity water containing sufficient dissolved oxygen (DO) become more than 2 orders of magnitude higher than that for bulk pure water. In this study effect of conductivity and DO concentration of bulk water on
crev) exposed to high temperature and high purity water containing sufficient dissolved oxygen (DO) become more than 2 orders of magnitude higher than that for bulk pure water. In this study effect of conductivity and DO concentration of bulk water on  crev of Type-316L stainless steel have been studied in 288
crev of Type-316L stainless steel have been studied in 288 C water. Following conclusion have been obtained: (1)
C water. Following conclusion have been obtained: (1)  crev increased with increasing DO concentration from 3 ppb to approximately 300 ppb. Above 300 ppb,
crev increased with increasing DO concentration from 3 ppb to approximately 300 ppb. Above 300 ppb,  crev did not simply increased with DO concentraion. (2) maximum
crev did not simply increased with DO concentraion. (2) maximum  crev was not affected by bulk water conductivity suggested that
crev was not affected by bulk water conductivity suggested that  crev would be determined by chemical equilibrium reaction. (3)
crev would be determined by chemical equilibrium reaction. (3)  crev-time curves were not affected by crevice depth. It was assumed that anion required to increase
crev-time curves were not affected by crevice depth. It was assumed that anion required to increase  crev generated within the crevice.
crev generated within the crevice.
Soma, Yasutaka; Komatsu, Atsushi; Igarashi, Takahiro; Yamamoto, Masahiro*
no journal, ,
Inside the stainless steel crevices including Stress Corrosion Cracking (SCC) exposed to oxygenated high-temperature water, an acidic, corrosive crevice environment is formed. Dissolved oxygen (DO) is supplied near the crevice mouth but depletes further inside, leading to a formation of the differential aeration cell and crevice environment. The precise location of the boundary between the bulk and crevice environments, known as the Electrochemical Crevice Mouth (ECM), is critical to provide a crevice corrosion criteria but not clearly defined. This study experimentally determined the ECM in Type-316L stainless steel by analyzing surface oxides formed in the crevice and the measurement of crevice solution's electrical conductivity at 288 C. The ECM location was identified by a change in surface oxide composition and a significant increase in solution conductivity. Over time, the ECM shifted deeper into the crevice, influenced by factors like DO levels and oxide layer growth. Numerical simulations supported these findings, suggesting that as the surface oxide layer inside the crevice grew, it decreased cathodic DO consumption, causing the ECM to move deeper into the crevice.
C. The ECM location was identified by a change in surface oxide composition and a significant increase in solution conductivity. Over time, the ECM shifted deeper into the crevice, influenced by factors like DO levels and oxide layer growth. Numerical simulations supported these findings, suggesting that as the surface oxide layer inside the crevice grew, it decreased cathodic DO consumption, causing the ECM to move deeper into the crevice.
Soma, Yasutaka; Kato, Chiaki; Ueno, Fumiyoshi
no journal, ,
To understand the effect of crevice solution chemistry on stress corrosion cracking of stainless steel in high temperature pure water, local solution electrical conductivity was measured using artificial crevice with small sensors. If the crevice gap was narrow enough, local solution electrical conductivity increased more than 100 times than that of bulk pure water and intergranular corrosion occurred.
Soma, Yasutaka; Kato, Chiaki; Ueno, Fumiyoshi
no journal, ,
Stress corrosion cracking (SCC) on stainless steels have been recognized as one of the most important corrosion-related failure in light water reactors. Many researches have been pointed out that the SCC advances under altered solution chemistry condition at the crack tip region compared to the bulk pure water. However, little works have been done to clarify degree of the alteration as function of bulk water condition, geometrical factor, and time. In this work, we carried out in-situ measurement of solution electrical conductivity within crevice of stainless steels. To create crevice specimen, a couple of stainless steel plate was fixed with bolts and nuts. Small sensors were imbedded into the crevice plate at three different positions with different crevice gaps. The crevice specimen with sensors was exposed to 288 C water with pressure of 8 MPa, dissolved oxygen concentration of 32 ppm. The solution electrical conductivity at the crevice gap of 6e-5 m was almost same to that of bulk pure water. At the crevice position with 1e-5 m gap, the maximum conductivity value was nearly 1000 times higher than that of bulk water and that is equivalent to decrease in pH of 3 from the neutral value. This indicates, if the crevice gap was narrow enough, local acidification occurred at the tip of the crevice.
C water with pressure of 8 MPa, dissolved oxygen concentration of 32 ppm. The solution electrical conductivity at the crevice gap of 6e-5 m was almost same to that of bulk pure water. At the crevice position with 1e-5 m gap, the maximum conductivity value was nearly 1000 times higher than that of bulk water and that is equivalent to decrease in pH of 3 from the neutral value. This indicates, if the crevice gap was narrow enough, local acidification occurred at the tip of the crevice.
Soma, Yasutaka; Ueno, Fumiyoshi; Yamamoto, Masahiro
no journal, ,
Diffusion behavior of dissolved oxygen into crevice of stainless steel in high temperature is very important to understand crevice environment. In this research, we developed 3D model of crevice and using it, we carried out numerical simulation of dissolved oxygen diffusion into the crevice. The result of numerical simulation showed good agreement with experimentally obtained result.
Soma, Yasutaka; Kato, Chiaki; Ueno, Fumiyoshi; Aoki, So; Inagaki, Hiromitsu*
no journal, ,
Crevice environment was measured by electrochemical sensors in high temperature pure water. Crevice environment and surface oxide layer on the crevice surface was analyzed in terms of crevice's geometrical factors (crevice gap, g and depth, d). The results were plotted on the g-d plane. It was shown that electrical conductivity of crevice solution was very high in oxygen depleted zone and the zone shrinked with increasing crevice gap, g.
Soma, Yasutaka; Ueno, Fumiyoshi; Inagaki, Hiromitsu*
no journal, ,
Effect of crevice geometry on corrosion environment within crevice of stainless steel in high temperature water was studied.