Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sato, Tetsuya; Nagame, Yuichiro*
Nihon Butsuri Gakkai-Shi, 78(2), p.64 - 72, 2023/02
The study of the chemistry of superheavy elements, which are located in the heavy extremes of the periodic table, has made considerable progress over the past 20 years, and new approaches based on various ideas have recently been developed. Research groups in Japan have also made significant contributions to the development of research on superheavy elements. Recently, notable results have been reported for the transactinide elements rutherfordium (element 104), dubnium (element 105), and seaborgium (element 106), and the heavy actinides with atomic numbers exceeding 100. The review will focus on the recent main results of these elements. This review outlines the main recent results and touches on future prospects.
Sato, Tetsuya
Kagaku To Kogyo, 72(10), P. 867, 2019/10
We conducted measurements of the first ionization potential (IP ) of the heavy actinide elements, lawrencium (Lr,
) of the heavy actinide elements, lawrencium (Lr, 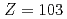 ), nobelium (No,
), nobelium (No, 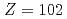 ), mendelevium (Md,
), mendelevium (Md, 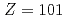 ) and fermium (Fm,
) and fermium (Fm, 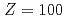 ) by using a novel method based on a surface ionization process. The IP
) by using a novel method based on a surface ionization process. The IP measurements have been performed using the ISOL (Isotope Separator On-Line) system equipped with a surface ion-source with short-lived heavy actinide isotopes,
measurements have been performed using the ISOL (Isotope Separator On-Line) system equipped with a surface ion-source with short-lived heavy actinide isotopes,  Lr (
Lr (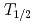 = 27s),
= 27s), 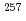 No (
No (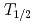 = 24.5s),
= 24.5s), 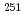 Md (
Md (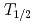 = 4.27 min), and
= 4.27 min), and  Fm (
Fm (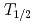 = 2.6 min). Our experimental results clearly showed that the IP
= 2.6 min). Our experimental results clearly showed that the IP of Lr is distinctly low among actinide elements. Moreover, No has the highest IP
of Lr is distinctly low among actinide elements. Moreover, No has the highest IP among them due to its full-filled 5f and 7s orbitals; the IP
among them due to its full-filled 5f and 7s orbitals; the IP value increased with an atomic number up to No and decreased dramatically at Lr, indicating the similar trend with that of heavy lanthanide elements. Therefore, we concluded Lr would be the last member of the actinide series.
value increased with an atomic number up to No and decreased dramatically at Lr, indicating the similar trend with that of heavy lanthanide elements. Therefore, we concluded Lr would be the last member of the actinide series.
Sato, Tetsuya; Asai, Masato; Borschevsky, A.*; Beerwerth, R.*; Kaneya, Yusuke*; Makii, Hiroyuki; Mitsukai, Akina*; Nagame, Yuichiro; Osa, Akihiko; Toyoshima, Atsushi; et al.
Journal of the American Chemical Society, 140(44), p.14609 - 14613, 2018/11
Times Cited Count:32 Percentile:69.73(Chemistry, Multidisciplinary)The first ionization potential (IP ) yields information on valence electronic structure of an atom. IP
) yields information on valence electronic structure of an atom. IP values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP
values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP
of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP
among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP
values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
Sato, Tetsuya; Asai, Masato; Borschevsky, A.*; Stora, T.*; Sato, Nozomi*; Kaneya, Yusuke; Tsukada, Kazuaki; D llmann, C. E.*; Eberhardt, K.*; Eliav, E.*; et al.
llmann, C. E.*; Eberhardt, K.*; Eliav, E.*; et al.
EPJ Web of Conferences, 131, p.05001_1 - 05001_6, 2016/12
Times Cited Count:0 Percentile:0.00(Chemistry, Inorganic & Nuclear)Ionization efficiency in a surface ionization process depends on the first ionization potential of the atom. Based on the dependence, the ionization potential of the atom can be determined. We measured ionization efficiencies of fermium, einsteinium, mendelevium, and lawrencium by using a newly developed gas-jet coupled surface ion-source. The ionization potential of the elements have not been determined so far due to their low production rates and/or their short half-lives. Based on a relationship between the ionization efficiency and the ionization potential obtained via measurements of short-lived lanthanide isotopes, the ionization potentials of these actinide elements have been successfully determined.
Sato, Tetsuya
Genshikaku Kenkyu, 61(1), p.96 - 106, 2016/09
We successfully determined the first ionization potential of lawrencium (Lr, Z=103). The result experimentally substantiated for the first time that Lr is the last member of the actinide series. Measured ionization potential suggested that Lr atom would have the electronic configuration which is different from the configuration expected based on the Periodic table. For the measurement, we have developed a novel method applied the surface ionization process. Public responses after the publication are also introduced.
Sato, Tetsuya
Kagaku, 71(3), p.12 - 16, 2016/03
We successfully confirmed that lawrencium, element 103, would be the last member of actinide series by a measurement of the first ionization potential of lawrencium. Moreover, the electronic configuration expected from the experimental results suggested that lawrencium could have the outermost electronic orbital similar to that of group-13 elements. Our result triggered discussion concerning the position of lawrencium and lutetium on the periodic table of the elements.
Sato, Tetsuya
Isotope News, (740), p.16 - 19, 2015/12
We successfully determined the first ionization potential of lawrencium (Lr, Z=103). The result experimentally substantiated for the first time that Lr is the last member of the actinide series. Measured ionization potential suggested that Lr atom would have the electronic configuration which is different from the configuration expected based on the Periodic table.
Sato, Tetsuya
Nihon Genshiryoku Gakkai-Shi ATOMO , 57(11), p.741 - 744, 2015/11
, 57(11), p.741 - 744, 2015/11
We have experimentally confirmed that Lr would be the last member of actinides series for the first time by a measurement of the first ionization potential of lawrencium (Lr, element 103). The electronic orbital of Lr atom which is estimated by the result suggests that Lr could have the outermost electronic orbital similar with group-13 elements. This work triggered a discussion concerning positions of Lr and lutetium, lanthanide homologue of Lr.
Sato, Tetsuya
Hosha Kagaku, (32), p.34 - 41, 2015/09
In the surface ionization process, an ionization efficiency depends on the first ionization potential of the atom of the element. The ionization potential can be estimated by using the relationship. This method has been developed in order to determine the first ionization potential of lawrencium (Lr, element 103). The value of the ionization potential of Lr have not been measured experimentally due to its low production rate and short half-life. The surface-ionization method is described in detail in this paper.
Sato, Tetsuya; Nagame, Yuichiro; Tsukada, Kazuaki
Kagaku To Kogyo, 68(9), p.824 - 826, 2015/09
We successfully confirmed that lawrencium (Lr, element 103) is the last member of actinide series by a measurement of its first ionization potential. Obtained experimental result suggested that the outermost electronic orbital of Lr atom would have p-orbital similar to elements of group-13. Our result triggered again the discussion of the position of Lr and lutetium, the lanthanide homologue of Lr, on the Periodic Table.
Sato, Tetsuya
Saiensu Potaru (Internet), 3 Pages, 2015/07
On April 9th, a press release titled "Measurement of the first ionization potential of lawrencium (element 103) - Unravelling Relativistic Effects in the Heaviest Actinide Element -" was issued. This research result published from Nature was not only introduced in its "News & Views" but also appeared on the cover. I made a commentary on the result and introduced its response for public.
Sato, Tetsuya; Asai, Masato; Borschevsky, A.*; Stora, T.*; Sato, Nozomi; Kaneya, Yusuke; Tsukada, Kazuaki; D llmann, Ch. E.*; Eberhardt, K.*; Eliav, E.*; et al.
llmann, Ch. E.*; Eberhardt, K.*; Eliav, E.*; et al.
Nature, 520(7546), p.209 - 211, 2015/04
Times Cited Count:112 Percentile:97.11(Multidisciplinary Sciences)Ionization efficiency in a surface ionization process depends on the first ionization potential of the atom. Based on the dependence, the ionization potential of the atom can be determined. We successfully measured ionization efficiencies of lawrencium (Lr, 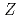 =103) using a gas-jet coupled surface ion-source. The ionization potential of Lr has not been determined owing to its low production rate and its short half-life. Based on a relationship between the ionization efficiency and the ionization potential obtained via measurements of short-lived lanthanide isotopes, the ionization potential of Lr was determined.
=103) using a gas-jet coupled surface ion-source. The ionization potential of Lr has not been determined owing to its low production rate and its short half-life. Based on a relationship between the ionization efficiency and the ionization potential obtained via measurements of short-lived lanthanide isotopes, the ionization potential of Lr was determined.
 F
F (n=2-5) clusters
(n=2-5) clustersHaketa, Naoki*; Yokoyama, Keiichi; Tanaka, Hiromasa*; Kudo, Hiroshi*
Journal of Molecular Structure; THEOCHEM, 577(1), p.55 - 67, 2002/01
no abstracts in English
Oshima, Takeshi; Uedono, Akira*; Abe, Koji*; Ito, Hisayoshi; Aoki, Yasushi; Yoshikawa, Masahito; Tanigawa, Shoichiro*; Nashiyama, Isamu
Applied Physics A, 67(4), p.407 - 412, 1998/00
Times Cited Count:28 Percentile:73.99(Materials Science, Multidisciplinary)no abstracts in English
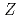 = 103)
= 103)Sato, Tetsuya; Asai, Masato; Kaneya, Yusuke; Tsukada, Kazuaki; Toyoshima, Atsushi; Miyashita, Sunao*; Oe, Kazuhiro*; Osa, Akihiko; Ichikawa, Shinichi; Nagame, Yuichiro; et al.
no journal, ,
The first ionization potentials of the heaviest actinide elements have not been measured until today owing to short half-lives and low production rates of the isotopes. Based on the surface ionization technique, we performed a measurement of the ionization potential of the heaviest actinide element, lawrencium (Lr, 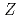 = 103), by using a newly developed surface ion-source installed to the JAEA-ISOL (Isotope Separator On-Line) at the JAEA tandem accelerator facility. We report on an evaluation of the IP value of Lr based on comparison of ionization behavior of
= 103), by using a newly developed surface ion-source installed to the JAEA-ISOL (Isotope Separator On-Line) at the JAEA tandem accelerator facility. We report on an evaluation of the IP value of Lr based on comparison of ionization behavior of  Lr with that of short-lived lanthanide isotopes on Ta surface at several temperature.
Lr with that of short-lived lanthanide isotopes on Ta surface at several temperature.
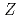 = 102)
= 102)Sato, Tetsuya; Asai, Masato; Kaneya, Yusuke; Tsukada, Kazuaki; Toyoshima, Atsushi; Vascon, A.; Takeda, Shinsaku; Mitsukai, Akina*; Nagame, Yuichiro; Ichikawa, Shinichi; et al.
no journal, ,
In order to determine the IP of the heavy elements, we have developed a novel measurement method based on a surface ionization technique by using a surface ionization ion source coupled to a He/CdI gas-jet transport system for an Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator facility. In this work, we have determined IP value of No by using the method. In a surface ionization process, an ionization efficiency of an atom depends on its IP. To obtain a relationship between IP and ionization efficiency in present system, we measured ionization efficiencies of various short-lived isotopes. Ionization efficiency of
gas-jet transport system for an Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator facility. In this work, we have determined IP value of No by using the method. In a surface ionization process, an ionization efficiency of an atom depends on its IP. To obtain a relationship between IP and ionization efficiency in present system, we measured ionization efficiencies of various short-lived isotopes. Ionization efficiency of 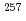 No produced in the
No produced in the  Cm(
Cm( C, 4n) reaction was also measured. Measured ionization efficiency of
C, 4n) reaction was also measured. Measured ionization efficiency of 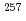 No was 0.8%, which yields IP value of No to be 6.6 eV. This value is in a good agreement with the value which has been evaluated by extrapolation from those of the lighter actinide elements, 6.65 eV.
No was 0.8%, which yields IP value of No to be 6.6 eV. This value is in a good agreement with the value which has been evaluated by extrapolation from those of the lighter actinide elements, 6.65 eV.
Sato, Tetsuya; Asai, Masato; Kaneya, Yusuke*; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Takeda, Shinsaku*; Vascon, A.*; Sakama, Minoru*; Sato, Daisuke*; et al.
no journal, ,
The first ionization potential (IP ) yields information on valence electronic structure of an atom. IP
) yields information on valence electronic structure of an atom. IP values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP
values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP
of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP
among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP
values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
Sato, Tetsuya
no journal, ,
The chemical and atomic properties of the heavy elements with atomic numbers Z  100, affected by strong relativistic effects, are studied at an-atom-at-a-time scale using the JAEA-ISOL. We successfully determined the first ionization potential (IP
100, affected by strong relativistic effects, are studied at an-atom-at-a-time scale using the JAEA-ISOL. We successfully determined the first ionization potential (IP ) values of heavy actinide elements from fermium (Fm, Z = 100) to lawrencium (Lr, Z = 103) by using a surface ion-source installed in the Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator facility. The adsorption behavior of Lr is being studied by a newly developed method combining vacuum chromatography with surface ionization in a metallic column/ionizer of the ISOL as well.
) values of heavy actinide elements from fermium (Fm, Z = 100) to lawrencium (Lr, Z = 103) by using a surface ion-source installed in the Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator facility. The adsorption behavior of Lr is being studied by a newly developed method combining vacuum chromatography with surface ionization in a metallic column/ionizer of the ISOL as well.
Sato, Tetsuya
no journal, ,
We measured the first ionization energy (IE ) of nobelium (No, Z = 102) and lawrencium (Lr, Z =103) by exploiting the dependence of the ionization efficiency (
) of nobelium (No, Z = 102) and lawrencium (Lr, Z =103) by exploiting the dependence of the ionization efficiency (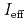 ) on the IE
) on the IE in a surface ionization process. The isotopes
in a surface ionization process. The isotopes 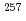 No (
No (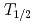 = 24.5s) and
= 24.5s) and  Lr (
Lr (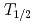 = 27 s), produced in the reaction
= 27 s), produced in the reaction  Cm (
Cm ( C, 4n) and
C, 4n) and  Cf (
Cf (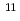 B, 4n), respectively, were used for studying their ionization. The reaction products recoiling from the targets were transported to a surface ion-source by a He/CdI
B, 4n), respectively, were used for studying their ionization. The reaction products recoiling from the targets were transported to a surface ion-source by a He/CdI gas-jet transport system. The products ionized in the ion-source were mass-separated with JAEA-ISOL. The number of ions collected at the end of the ISOL was determined by
gas-jet transport system. The products ionized in the ion-source were mass-separated with JAEA-ISOL. The number of ions collected at the end of the ISOL was determined by  -particle measurements and was used to evaluate
-particle measurements and was used to evaluate 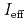 values. With the present system, we successfully ionized and mass-separated
values. With the present system, we successfully ionized and mass-separated 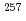 No and
No and  Lr with efficiencies of (0.5
Lr with efficiencies of (0.5  0.1)% and (36
0.1)% and (36  7)% at 2800 K, respectively. From these
7)% at 2800 K, respectively. From these 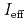 values, IE
values, IE values of No and Lr were determined based on the relationship between
values of No and Lr were determined based on the relationship between 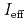 and IE
and IE . Our values are in good agreement with the predicted ones by theoretical calculations.
. Our values are in good agreement with the predicted ones by theoretical calculations.
Sato, Tetsuya
no journal, ,
Ionization efficiency in a surface ionization process depends on the first ionization potential of the atom. Based on the dependence, the ionization potential of the atom can be determined. We measured ionization efficiencies of fermium, einsteinium, mendelevium, and lawrencium by using a newly developed gas-jet coupled surface ion-source. The ionization potential of the elements have not been determined so far due to their low production rates and/or their short half-lives. Based on a relationship between the ionization efficiency and the ionization potential obtained via measurements of short-lived lanthanide isotopes, the ionization potentials of these actinide elements have been successfully determined.