Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 decay of
decay of  In;
In;  emission from neutron-unbound states in
emission from neutron-unbound states in  Sn
SnPiersa, M.*; Korgul, A.*; Benito, J.*; Andreyev, A. N.; 75 of others*
Physical Review C, 99(2), p.024304_1 - 024304_10, 2019/02
Times Cited Count:11 Percentile:73.49(Physics, Nuclear)Hemmi, Ko
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 23(1), P. 113, 2016/06
no abstracts in English
Hemmi, Ko; Yamaguchi, Tetsuji; Iida, Yoshihisa
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 22(1), p.3 - 10, 2015/06
Iodine and tin are important elements in performance assessment of geological disposal of radioactive wastes. Sorption experiments of iodine were carried out under varying nitrate concentration with a range of 0 to 5 mol dm at neutral pH range in order to determine the distribution coefficient of iodine was zero or non-zero value. The experimental results with estimated statistical errors showed non-zero values for tuffaceous sandstone except for NaNO
at neutral pH range in order to determine the distribution coefficient of iodine was zero or non-zero value. The experimental results with estimated statistical errors showed non-zero values for tuffaceous sandstone except for NaNO concentration 0.5 mol dm
concentration 0.5 mol dm . Non-zero values were also obtained under NaNO
. Non-zero values were also obtained under NaNO concentrations higher than 0.5 mol dm
concentrations higher than 0.5 mol dm for granodiorite. Sorption experiments of tin were carried out at high pH range in order to check whether the distribution coefficient of tin decreases significantly with pH as a result of formation of anionic hydrolysis species of tin. The distribution coefficients of tin on granodiorite decreased from 9.79
for granodiorite. Sorption experiments of tin were carried out at high pH range in order to check whether the distribution coefficient of tin decreases significantly with pH as a result of formation of anionic hydrolysis species of tin. The distribution coefficients of tin on granodiorite decreased from 9.79 10
10 m
m kg
kg at pH10.4 to 2.46
at pH10.4 to 2.46 10
10 m
m kg
kg at pH12.4. The distribution coefficient of tin on tuffaceous sandstone was about one order of magnitude higher (about 2
at pH12.4. The distribution coefficient of tin on tuffaceous sandstone was about one order of magnitude higher (about 2 10
10 m
m kg
kg ) than that of granodiorite at pH around 12.4.
) than that of granodiorite at pH around 12.4.

Fuchizaki, Kazuhiro*; Fujii, Yasuhiko*; Oishi, Yasuo*; Omura, Ayako*; Hamaya, Nozomu*; Katayama, Yoshinori; Okada, Taku
Journal of Chemical Physics, 120(23), p.11196 - 11199, 2004/06
Times Cited Count:22 Percentile:58.10(Chemistry, Physical)The location of the liquidus in the low-pressure crystalline phase of SnI was determined utilizing
was determined utilizing  X-ray diffraction measurements under pressures up to approximately
X-ray diffraction measurements under pressures up to approximately 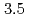 GPa. The liquidus is not well fitted to a monotonically increasing curve such as Simon's equation, but breaks near
GPa. The liquidus is not well fitted to a monotonically increasing curve such as Simon's equation, but breaks near  GPa and then becomes almost flat. The results are compared to those from molecular dynamics simulations. Ways to improve the model potential adopted in the simulations are discussed.
GPa and then becomes almost flat. The results are compared to those from molecular dynamics simulations. Ways to improve the model potential adopted in the simulations are discussed.
 determined by in situ X-ray observations up to 30GPa and 1400K
determined by in situ X-ray observations up to 30GPa and 1400KOno, Shigeaki*; Ito, Eiji*; Katsura, Tomoo*; Yoneda, Akira*; Walter, M.*; Urakawa, Satoru*; Utsumi, Wataru; Funakoshi, Kenichi*
Physics and Chemistry of Minerals, 27(9), p.618 - 622, 2000/11
Times Cited Count:44 Percentile:79.77(Materials Science, Multidisciplinary)no abstracts in English
Tamada, Masao; Koshikawa, Hiroshi; Hosoi, Fumio; Suwa, Takeshi; Usui, H.*; ; Sato, H.*
Polymer, 40(1), p.3061 - 3067, 1999/00
no abstracts in English
Shigeta, Noriko; Matsuoka, Hiromitsu; Sekine, Toshiaki; Osa, Akihiko; Koizumi, Mitsuo; ; ; ; ; Lambrecht, R. M.*
Dai-4-Kai TIARA Kenkyu Happyokai Yoshishu, p.70 - 71, 1995/06
no abstracts in English
; Kato, Kaneharu
Bunseki Kagaku, 39, p.533 - 538, 1990/00
no abstracts in English



 Sn
SnOzawa, K.;
Physica Status Solidi, 40(2), p.K199 - K202, 1977/02
no abstracts in English
JAERI-M 6669, 19 Pages, 1976/08
no abstracts in English
;
Bunseki Kagaku, 20(8), p.970 - 974, 1971/00
no abstracts in English
; ;
Nihon Kinzoku Gakkai-Shi, 31(8), p.993 - 998, 1967/00
no abstracts in English


 Sn in aqueous solution with tin metal
Sn in aqueous solution with tin metal;
Nihon Genshiryoku Gakkai-Shi, 4(10), p.713 - 715, 1962/00
no abstracts in English
Ebihara, Kenichi; Fujihara, Hiro*; Shimizu, Kazuyuki*; Yamaguchi, Masatake; Toda, Hiroyuki*
no journal, ,
Hydrogen embrittlement (HE) is an inevitable problem in strengthening aluminum alloys. In the alloys, H diffusively segregates into interfacial defects such as grain boundaries and phase interfaces, weakening the atomic bonds there and causing embrittlement. Hence there is a possibility to suppress HE by reducing the interfacial segregation of H. Recently, atomic-level calculations have revealed that a second-phase particle of tin (Sn) in aluminum alloys can trap H in its interior. Furthermore, suppression of HE has been reported experimentally in tin-doped aluminum alloys. In this study, a code for simulating H diffusion in 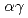 duplex steels was applied to H diffusion in aluminum containing a Sn second-phase particle to evaluate the possibility of H penetration into the particle according to the experimental conditions. As a result, it was confirmed that some amount of H can penetrate into the particles. This contributes to the verification of the experiment by simulation.
duplex steels was applied to H diffusion in aluminum containing a Sn second-phase particle to evaluate the possibility of H penetration into the particle according to the experimental conditions. As a result, it was confirmed that some amount of H can penetrate into the particles. This contributes to the verification of the experiment by simulation.
Ito, Miki*; Akagi, Yosuke*; Kitamura, Akira
no journal, ,
Distribution coefficient of Sn onto Na-type and Ca-type montmorillonite samples was determined under high-pH conditions with a batch method. It was suggested that sorption behavior of Sn under cementitious conditions was affected through changing major aqueous species accompanied with pH change.
Ichihara, Hirotsugu*; Katsuki, Kenta*; Arai, Tsuyoshi*; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki
no journal, ,
no abstracts in English