Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nagasaki, Shinya*
JNC TJ8400 2000-004, 32 Pages, 2000/02
Equilibrium and kinetics of sorption of NpO on illite were investigated at pH = 6 by using the differential pulse anodic stripping voltammetry method and the spectroscopic method, respectively. It was found that the sorption isotherm obtained was fitted better by the Langmuir-Freundlich type equation than by the Langmuir equation. The heterogeneity coefficient was 0.89
on illite were investigated at pH = 6 by using the differential pulse anodic stripping voltammetry method and the spectroscopic method, respectively. It was found that the sorption isotherm obtained was fitted better by the Langmuir-Freundlich type equation than by the Langmuir equation. The heterogeneity coefficient was 0.89  0.05 and the half width at half maximum (HWHM) of affinity spectrum was 0.19 log unit, indicating that the surfacc of illite used has a low degree of heterogeneity. The kinetic spectra indicated that the sorption of NpO
0.05 and the half width at half maximum (HWHM) of affinity spectrum was 0.19 log unit, indicating that the surfacc of illite used has a low degree of heterogeneity. The kinetic spectra indicated that the sorption of NpO occurs only at the outer surfacc. The mean HWHM of the kinetic spectra was 0.18 log unit. This also proves that the sorption kinetics of NpO
occurs only at the outer surfacc. The mean HWHM of the kinetic spectra was 0.18 log unit. This also proves that the sorption kinetics of NpO on the illite used is controlled by the same heterogeneity of the sorption sites. From the dependence of mean rate constants on temperature, a mean apparent activation enthalpy and a mean apparent activation entropy were evaluated at 37
on the illite used is controlled by the same heterogeneity of the sorption sites. From the dependence of mean rate constants on temperature, a mean apparent activation enthalpy and a mean apparent activation entropy were evaluated at 37 3 kJ/mol and - 69
3 kJ/mol and - 69  7 J/K
7 J/K mol, respectively. This value of enthalpy suggests that the sorption is not controlled by diffusion through the hydrodynamic film around the illite. Equilibrium and kinetics of sorption of NpO
mol, respectively. This value of enthalpy suggests that the sorption is not controlled by diffusion through the hydrodynamic film around the illite. Equilibrium and kinetics of sorption of NpO and Np(V) carbonate complexes (mainly NpO
and Np(V) carbonate complexes (mainly NpO CO
CO ) on Na-montmorillonite were also examined by using same technique.
) on Na-montmorillonite were also examined by using same technique.

 and AnO
and AnO
 Species in Geologic Environments
Species in Geologic EnvironmentsChoppin, G. R.*; Bronikowski, M.*; Chen, J.*; Byegard, J.*; Rai, D.*; Yui, Mikazu
JNC TN8400 99-012, 155 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of pentavalent and hexavalent actinide species (AnO and AnO
and AnO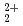 ) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO
) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO and AnO
and AnO
 , redox potentials and equilibrium constants of redox reactions for actinides are also included.
, redox potentials and equilibrium constants of redox reactions for actinides are also included.
Suzuki, Atsuyuki*; Nagasaki, Shinya*
PNC TJ1602 92-001, 75 Pages, 1992/03
For the safety assessment of high level waste disposal system, it is important to understand the migration behaviors of actinides in the near- or far-field. Although the importance of migration of actinides which form the colloids has been pointed out recently, the migration behaviors of colloids are not fully understood. In this study, the migration behaviors of colloids were investigated experimentally by column method. In the experiments, quartz powder was used as the solid. Ferric hydrous oxide colloids were prepared as the real colloids and neptunium(V) formed Np(V)-Fe(III) pseudocolloids by sorption. Some fractions of the colloids eluted with the eluting solution. Also observed were strongly retarded fractions. Non-negligible fractions were found to migrate faster than the eluting solution. These migration characteristics were qualitatively understood in this work.