Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 particles triggered strong interaction with LaFeO
particles triggered strong interaction with LaFeO framework for total and preferential CO oxidation
framework for total and preferential CO oxidationZheng, Y.*; Xiao, H.*; Li, K.*; Wang, Y.*; Li, Y.*; Wei, Y.*; Zhu, X.*; Li, H.-W.*; Matsumura, Daiju; Guo, B.*; et al.
ACS Applied Materials & Interfaces, 12(37), p.42274 - 42284, 2020/09
Times Cited Count:27 Percentile:70.49(Nanoscience & Nanotechnology)Nagai, Takayuki; Sato, Nobuaki*; Sasage, Kenichi
Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten Kenkyu Seika Hokokusho (Heisei-27-Nendo) (CD-ROM), 2 Pages, 2016/03
no abstracts in English
Yamazaki, Satoshi*; Yamashita, Toshiyuki; Matsui, Tsuneo*; Nagasaki, Takanori*
Journal of Nuclear Materials, 294(1-2), p.183 - 187, 2001/04
Times Cited Count:43 Percentile:92.51(Materials Science, Multidisciplinary)no abstracts in English
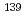 La NMR relaxation and chemical shift studies in the aqueous nitrate and chloride Solutions
La NMR relaxation and chemical shift studies in the aqueous nitrate and chloride SolutionsYaita, Tsuyoshi; ; Tachimori, Shoichi
Journal of Physical Chemistry B, 102(20), p.3886 - 3891, 1998/00
Times Cited Count:26 Percentile:61.38(Chemistry, Physical)no abstracts in English
Kimura, Takaumi; Choppin, G. R.*; ; Yoshida, Zenko
Radiochimica Acta, 72, p.61 - 64, 1996/00
no abstracts in English
Shibata, Atsuhiro; P.Y.COR*; ; ; ; ; Tanaka, Yasumasa
PNC TN8410 95-112, 100 Pages, 1995/05
PNC has developed the TRUEX process to recover transuranium elements from high level liquid waste. This process uses CMPO as extracting solvent. DIAMEX process which uses Diamide has been developed bY CEA in France. The aim of basic studies was to get some comparison data between CMPO and Diamide. We have conducted the experiments regarding properties of Ln(III) extraction and behavior of third phase formation. Also, we have investigated properties of Ln(III) extraction with TBP and DBBP to compare bidentate ligand with monodentate ligand. This study was conducted as a frame of PNC/CEA collaboration on minor Actinide partitioning. The conclusions drawn from our studies can be summarized as follows; (1)Trivalent Lanthanides extraction reaction. CMPO, TBP and DBBP ; Ln + 3NO
+ 3NO + 3Extractant
+ 3Extractant 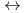 Ln(NO
Ln(NO )
)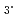 3Extractant. Diamide; Ln
3Extractant. Diamide; Ln + 3NO
+ 3NO + m Diamide
+ m Diamide 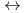 Ln(NO
Ln(NO )
)
 m Diamide : m=O.7
m Diamide : m=O.7 1.8. (2)Extraction equilibrium constant. Apparent extraction equilibrium constants were calculated from the trivalent Lanthanides extraction reaction. The value of the constants were CMPO
1.8. (2)Extraction equilibrium constant. Apparent extraction equilibrium constants were calculated from the trivalent Lanthanides extraction reaction. The value of the constants were CMPO  Diamide
Diamide 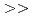 DBBP
DBBP  TBP.CMPO has high extraction efficiency for trivalent metals such as Am, but the back extraction is difficult in nitric acid media. Diamide indicated better behavior about both extraction and back extraction. (3)Separation Factors. We got separation factors (SFs) for intra-group in nitric acid. So SFs for CMPO and Diamide were nearly one, that these solvents could not separate each Lanthanides elements. The other hand, SFs for TBP and DBBP were 2
TBP.CMPO has high extraction efficiency for trivalent metals such as Am, but the back extraction is difficult in nitric acid media. Diamide indicated better behavior about both extraction and back extraction. (3)Separation Factors. We got separation factors (SFs) for intra-group in nitric acid. So SFs for CMPO and Diamide were nearly one, that these solvents could not separate each Lanthanides elements. The other hand, SFs for TBP and DBBP were 2  10. (4)Behavior of third phase formation. When using CMPO or Diamide, even in the case of extraction of nitric acid, third phase formation was found. When third phase was formed, the nitric acid concentration in aqueous phase was 6 N for CMPO, 4 N for Diamide. If Diamide diluted with Decaline was used, third phase wasn't found ...
10. (4)Behavior of third phase formation. When using CMPO or Diamide, even in the case of extraction of nitric acid, third phase formation was found. When third phase was formed, the nitric acid concentration in aqueous phase was 6 N for CMPO, 4 N for Diamide. If Diamide diluted with Decaline was used, third phase wasn't found ...
Kimura, Takaumi; Choppin, G. R.*
Journal of Alloys and Compounds, 213-214, p.313 - 317, 1994/00
Times Cited Count:273 Percentile:99.70(Chemistry, Physical)no abstracts in English
 solid solutions,II; Magnetic susceptibilities of solid solutions with high lanthanum and oxygen concentrations
solid solutions,II; Magnetic susceptibilities of solid solutions with high lanthanum and oxygen concentrationsHinatsu, Yukio
Journal of Solid State Chemistry, 95, p.300 - 306, 1991/00
Times Cited Count:8 Percentile:38.54(Chemistry, Inorganic & Nuclear)no abstracts in English


 solid solutions
solid solutions; Fujino, Takeo
Journal of Solid State Chemistry, (68), p.255 - 265, 1987/00
no abstracts in English
Fujino, Takeo; Tagawa, Hiroaki
Analytica Chimica Acta, 107, p.365 - 371, 1979/00
Times Cited Count:3no abstracts in English
; ; ;
Bunko Kenkyu, 18(5), p.262 - 267, 1969/00
no abstracts in English
; ; ;
Bunko Kenkyu, 18(4), p.210 - 217, 1969/00
no abstracts in English
 coated by 2D materials
coated by 2D materialsOgawa, Shuichi*; Yusa, Ryunosuke*; Wang, G.*; Pettes, M. T.*; Liu, F.*; Tsuda, Yasutaka; Yoshigoe, Akitaka; Abukawa, Tadashi*; Moody, N. A.*; Yamaguchi, Hisato*
no journal, ,
Lanthanum hexaboride (LaB ) has a low work-function and is widely used as a thermionic cathode. For practical application, further reduction of its work-function and high durability have been required. In this study, the effect of 2D material coating materials (graphene and hexagonal boron nitride (hBN)) prepared by a wet-transfer method on the work-function of LaB
) has a low work-function and is widely used as a thermionic cathode. For practical application, further reduction of its work-function and high durability have been required. In this study, the effect of 2D material coating materials (graphene and hexagonal boron nitride (hBN)) prepared by a wet-transfer method on the work-function of LaB (100) was studied by using photoelectron emission microscopy (PEEM), synchrotron radiation photoemission spectroscopy, Raman spectroscopy, atomic force microscopy and DFT calculations. PEEM images for samples after 905
(100) was studied by using photoelectron emission microscopy (PEEM), synchrotron radiation photoemission spectroscopy, Raman spectroscopy, atomic force microscopy and DFT calculations. PEEM images for samples after 905 C heating clearly showed strong photoemission in the hBN coating region. DFT calculations indicated that the work-function increases in graphene due to the inward dipole formation, while the work function decreases in hBN due to the outward dipole forming at the interface.
C heating clearly showed strong photoemission in the hBN coating region. DFT calculations indicated that the work-function increases in graphene due to the inward dipole formation, while the work function decreases in hBN due to the outward dipole forming at the interface.
 generation by reaction with Ru-La-Na mix nitrates and raw materials for vitrification
generation by reaction with Ru-La-Na mix nitrates and raw materials for vitrificationNagai, Takayuki; Kobayashi, Hidekazu; Okamoto, Yoshihiro; Sato, Nobuaki*; Inose, Takehiko*; Sato, Seiichi*; Hatakeyama, Kiyoshi*; Seki, Katsumi*
no journal, ,
It is thought that a generated ruthenium compound grows from a high level radioactive liquid waste into RuO crystal by reacting to raw materials for the vitrification process. In this study, the generation reaction to RuO
crystal by reacting to raw materials for the vitrification process. In this study, the generation reaction to RuO was confirmed by heating Ru-La-Na mix nitrates and the raw materials.
was confirmed by heating Ru-La-Na mix nitrates and the raw materials.