Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O)
O) ]
] with nitrate ions by using density functional theory calculation
with nitrate ions by using density functional theory calculationKato, Akane*; Kaneko, Masashi; Nakashima, Satoru*
RSC Advances (Internet), 10(41), p.24434 - 24443, 2020/06
Times Cited Count:6 Percentile:30.44(Chemistry, Multidisciplinary)Complexation reactions of ruthenium-nitrosyl complexes in HNO solution were investigated by density functional theory (DFT) calculations in order to predict the stability of Ru species in high-level radioactive liquid waste (HLLW) solution. Equilibrium structure of [Ru(NO)(NO
solution were investigated by density functional theory (DFT) calculations in order to predict the stability of Ru species in high-level radioactive liquid waste (HLLW) solution. Equilibrium structure of [Ru(NO)(NO )
) (H
(H O)
O) ] obtained by DFT calculations reproduced the experimental Ru-ligands bond lengths and IR frequencies reported previously. Comparison of the Gibbs energies among the geometrical isomers revealed that the complexation reactions of the ruthenium-nitrosyl complexes with NO
] obtained by DFT calculations reproduced the experimental Ru-ligands bond lengths and IR frequencies reported previously. Comparison of the Gibbs energies among the geometrical isomers revealed that the complexation reactions of the ruthenium-nitrosyl complexes with NO
 proceed via the NO
proceed via the NO
 coordination to the equatorial plane toward the Ru-NO axis. We also estimated Gibbs energy differences on the stepwise complexation reactions to succeed in reproducing the fraction of Ru-NO species in 6 M HNO
coordination to the equatorial plane toward the Ru-NO axis. We also estimated Gibbs energy differences on the stepwise complexation reactions to succeed in reproducing the fraction of Ru-NO species in 6 M HNO solution, such as in HLLW, by considering the association energy between the Ru-NO species and the substituting ligands. Electron density analyses of the complexes indicated that the strength of the Ru-ligands coordination bonds depends on the stability of the Ru species and the Ru complex without NO
solution, such as in HLLW, by considering the association energy between the Ru-NO species and the substituting ligands. Electron density analyses of the complexes indicated that the strength of the Ru-ligands coordination bonds depends on the stability of the Ru species and the Ru complex without NO
 at the axial position is more stable than that wit NO
at the axial position is more stable than that wit NO
 , which might attribute to the difference in the trans influence between H
, which might attribute to the difference in the trans influence between H O and NO
O and NO
 . Finally, we demonstrated the complexation kinetics in the reactions
. Finally, we demonstrated the complexation kinetics in the reactions 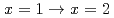 . The present study is expected to enable us to model the precise complexation reactions of platinum-group metals in HNO
. The present study is expected to enable us to model the precise complexation reactions of platinum-group metals in HNO solution.
solution.
 Ru M
Ru M ssbauer parameters of ruthenium-nitrosyl complexes
ssbauer parameters of ruthenium-nitrosyl complexesKaneko, Masashi; Kato, Akane*; Nakashima, Satoru*; Kitatsuji, Yoshihiro
Inorganic Chemistry, 58(20), p.14024 - 14033, 2019/10
Times Cited Count:12 Percentile:62.43(Chemistry, Inorganic & Nuclear)We applied density functional theory calculations to ruthenium-nitrosyl complexes, which are known to exist in high-level radioactive waste, to give a theoretical correlation between  Ru M
Ru M ssbauer spectroscopic parameters (
ssbauer spectroscopic parameters ( and
and 
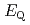 ) and ligand field strength (
) and ligand field strength (
 ) for the first time. The structures of the series of complexes, [Ru(NO)L
) for the first time. The structures of the series of complexes, [Ru(NO)L ] (L = Br
] (L = Br , Cl
, Cl , NH
, NH , CN
, CN ), were modeled based on the corresponding single-crystal X-ray coordinates. The comparisons of the geometries and total energies between the different spin states suggested that the singlet spin state of [Ru(II)(NO
), were modeled based on the corresponding single-crystal X-ray coordinates. The comparisons of the geometries and total energies between the different spin states suggested that the singlet spin state of [Ru(II)(NO )L
)L ] complexes were the most stable. The calculated results of both the
] complexes were the most stable. The calculated results of both the  and
and 
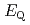 values reproduced the experimental results by reported previously and increased in the order of L = Br
values reproduced the experimental results by reported previously and increased in the order of L = Br , Cl
, Cl , NH
, NH , CN
, CN . Finally, we estimated the ligand field strength (
. Finally, we estimated the ligand field strength (
 ) based on molecular orbitals, assuming C
) based on molecular orbitals, assuming C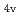 symmetry and showed the increase of
symmetry and showed the increase of 
 values in that order, being consistent with well-known spectrochemical series of ligands. The increase attributes to the strengthening of the abilities of
values in that order, being consistent with well-known spectrochemical series of ligands. The increase attributes to the strengthening of the abilities of  -donor and
-donor and  -acceptor of the L-ligands to the Ru atom, resulting in the increase of the
-acceptor of the L-ligands to the Ru atom, resulting in the increase of the  values.
values.
Kaneko, Masashi; Kato, Akane*; Nakashima, Satoru*; Kitatsuji, Yoshihiro; Watanabe, Masayuki
no journal, ,
Ruthenium exists as ruthenium nitrosyls, [Ru(NO)] , in high-level radioactive liquid waste and shows various stabilities depending on concentrations of nitrate and hydroxide ions. The detailed stabilities, however, remain to be unclear. As the first step to understand the stabilities of ruthenium nitrosyls, the present study focuses on the structural and bonding properties of the nitrosylruthenium species with fundamental ligands, such as chloride ion and ammonia. We modeled the ruthenium species by referring the corresponding single crystal structures and calculated the stable geometries under aqueous condition. The result reproduced the ruthenium-ligand bond lengths and the stretching vibrational energies of nitrosyl group. We also estimated
, in high-level radioactive liquid waste and shows various stabilities depending on concentrations of nitrate and hydroxide ions. The detailed stabilities, however, remain to be unclear. As the first step to understand the stabilities of ruthenium nitrosyls, the present study focuses on the structural and bonding properties of the nitrosylruthenium species with fundamental ligands, such as chloride ion and ammonia. We modeled the ruthenium species by referring the corresponding single crystal structures and calculated the stable geometries under aqueous condition. The result reproduced the ruthenium-ligand bond lengths and the stretching vibrational energies of nitrosyl group. We also estimated  Ru M
Ru M ssbauer isomer shifts based on electron density analysis and succeeded in reproducing the ismoer shifts. In the presentation, we will indicate the correlation between the isomer shifts and ligand field splitting derived by molecular orbital analysis and discuss an origin of the stability of ruthenium nitrosyls.
ssbauer isomer shifts based on electron density analysis and succeeded in reproducing the ismoer shifts. In the presentation, we will indicate the correlation between the isomer shifts and ligand field splitting derived by molecular orbital analysis and discuss an origin of the stability of ruthenium nitrosyls.
 Ru M
Ru M ssbauer isomer shift
ssbauer isomer shiftKaneko, Masashi; Kato, Akane*; Nakashima, Satoru*; Kitatsuji, Yoshihiro
no journal, ,
A linear relationship between M ssbauer isomer shift values and electron densities at nucleus position assures the quantitative evaluation of the covalent interaction between a M
ssbauer isomer shift values and electron densities at nucleus position assures the quantitative evaluation of the covalent interaction between a M ssbauer element and its surroundings. The use of this linear relationship makes us to predict a covalency in unknown compounds by electron density analysis based on quantum chemical calculations. As the first step to elucidate stability of ruthenium, which is known to be one of obstructive factors for separation process of high-level radioactive waste, in this study, we performed a fundamental investigation to predict the chemical bonding properties of nitrosylruthenium complexes. We modeled several nitrosylruthenium complexes with basic ligands, such chloride ion and ammonia, namely, [Ru(NO)L
ssbauer element and its surroundings. The use of this linear relationship makes us to predict a covalency in unknown compounds by electron density analysis based on quantum chemical calculations. As the first step to elucidate stability of ruthenium, which is known to be one of obstructive factors for separation process of high-level radioactive waste, in this study, we performed a fundamental investigation to predict the chemical bonding properties of nitrosylruthenium complexes. We modeled several nitrosylruthenium complexes with basic ligands, such chloride ion and ammonia, namely, [Ru(NO)L ] (L = Br
] (L = Br , Cl
, Cl , NH
, NH , CN
, CN ), by density functional calculation and estimated
), by density functional calculation and estimated  Ru M
Ru M ssbauer isomer shifts by electron density analysis. The calculated results reproduced the experimental metal-ligand bond lengths and M
ssbauer isomer shifts by electron density analysis. The calculated results reproduced the experimental metal-ligand bond lengths and M ssbauer isomer shifts within the error of 0.1 mm/s. We also calculated the orbital splitting values of Ru d-electron. The value increased in the order of L = Br
ssbauer isomer shifts within the error of 0.1 mm/s. We also calculated the orbital splitting values of Ru d-electron. The value increased in the order of L = Br , Cl
, Cl , NH
, NH , CN
, CN and correlated to that of M
and correlated to that of M ssbauer isomer shift values. This indicated that the covalent interation between metal and ligand originates in the d-electron contribution of ruthenium to the coordination bonds.
ssbauer isomer shift values. This indicated that the covalent interation between metal and ligand originates in the d-electron contribution of ruthenium to the coordination bonds.
Kato, Akane*; Kaneko, Masashi; Nakashima, Satoru*
no journal, ,
Ruthenium is considered to exist as nitrosylruthenium nitrate complexes in nitric acid such as high-level radioactive liquid waste. We estimated the structures of ruthenium complexes by using density functional theory calculation. The structures were modeled as [Ru(NO)(NO )
) (H
(H O)
O)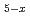 ] (x = 1-4) with octahedral coordination sphere by using its analogous single-crystal structure and molecular modeling software. As the result, the geometries in which nitrate ions coordinate to the equatrial position of Ru-NO axis were thermodynamically the most stable for all the complexes with x = 1-4.
] (x = 1-4) with octahedral coordination sphere by using its analogous single-crystal structure and molecular modeling software. As the result, the geometries in which nitrate ions coordinate to the equatrial position of Ru-NO axis were thermodynamically the most stable for all the complexes with x = 1-4.
Kato, Akane*; Kaneko, Masashi; Nakashima, Satoru*
no journal, ,
Aiming to predict ruthenium spices in high-level radioactive liquid waste, we demonstrated to simulate the complexation reaction between nitrosylruthenium and nitrate ions. When comparing the thermodynamic stability of the twelve geometrical isomers of the nitrosylruthenium nitrate complexes, the complexes with nitrate ions at the equatrial position toward the Ru-NO axis were confirmed to be the most stable isomers. Moreover, the Gibbs free energies analysis of the stepwise complexation reactions indicated that the Gibbs energy difference was improved the dependency of the fraction of the ruthenium species on the nitrate ions concentration by considering the association energy between the complex and the substituting ligands.