Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hayashi, Masaaki*; Nakahara, Hirotaka*; Shirakura, Shota*; Yamano, Hidemasa
Proceedings of International Conference on Nuclear Fuel Cycle (GLOBAL2024) (Internet), 4 Pages, 2024/10
As part of the development of risk assessment technologies for sodium-cooled fast reactor coupled to thermal energy storage (TES) system with sodium-molten salt heat exchanger (HX), simple evaluation of heat transfer performance using heat transfer coefficient formula is performed. And Computational Fluid Dynamics (CFD) thermal analyses by STAR-CCM+ with partial HX model are performed to develop evaluation technology. The performance evaluation technology of a HX between sodium and molten salt and the confirmation of heat transfer improvement measures effects is developed.
Yamano, Hidemasa; Takano, Kazuya; Kurisaka, Kenichi; Kikuchi, Shin; Kondo, Toshiki; Umeda, Ryota; Sato, Rika; Shirakura, Shota*
Dai-28-Kai Doryoku, Enerugi Gijutsu Shimpojiumu Koen Rombunshu (Internet), 5 Pages, 2024/06
This project studies investigation on safety design guideline and risk assessment technology for sodium-cooled fast reactor with the molten-salt heat storage system, development of evaluation method for heat transferring performance between sodium and molten-salt and improvement of the performance, and evaluation of chemical reaction characteristic between sodium and molten-salt and improvement of its safety. This paper describes the effect of sodium-molten salt heat transfer tube failure in addition to the project overview and progress.
Hayashi, Masaaki*; Nakahara, Hirotaka*; Abe, Takashi*; Matsunaga, Suhei*; Miyata, Hajime*; Shirakura, Shota*; Yamano, Hidemasa
Dai-28-Kai Doryoku, Enerugi Gijutsu Shimpojiumu Koen Rombunshu (Internet), 5 Pages, 2024/06
This paper describes the study of the performance evaluation technology of a heat exchanger between sodium and molten salt and the confirmation of heat transfer improvement measures effects up to FY2023.
Yamamoto, Yuri*; Minowa, Kazuki*; Takahatake, Yoko; Watanabe, So; Nakamura, Masahiro; Matsuura, Haruaki*
Electrochemistry (Internet), 92(4), p.043019_1 - 043019_4, 2024/04
Times Cited Count:0 Percentile:0.00(Electrochemistry)In the pyro-reprocessing of spent nuclear fuel, salt bath is normally used in several times, but at final moment, spent salt containing small amount of chloride nuclear fuel material is generated. In terms of managing nuclear fuel materials, it is desirable that the nuclear fuel materials should be recovered from the spent salt. We are proposing methods for recovering nuclear fuel materials using precipitation by oxide addition and distillation for reducing pressure techniques. In this study, we have focused on the behavior of manganese(II), which is one of the radioactivated products. As a result of experiments and thermodynamic simulation, it was found that manganese(II) is likely to be entrained in nuclear fuel materials. Therefore, it is necessary to add a step to separate manganese(II) from nuclear fuel materials.
Yamano, Hidemasa; Kurisaka, Kenichi; Takano, Kazuya; Kikuchi, Shin; Kondo, Toshiki; Umeda, Ryota; Shirakura, Shota*
Dai-27-Kai Doryoku, Enerugi Gijutsu Shimpojiumu Koen Rombunshu (Internet), 5 Pages, 2023/09
This project studies investigation on safety design guideline and risk assessment technology for sodium-cooled fast reactor with the molten-salt heat storage system, development of evaluation method for heat transferring performance between sodium and molten-salt and improvement of the performance, and evaluation of chemical reaction characteristic between sodium and molten-salt and improvement of its safety. The project overview is presented in this report.
Yamano, Hidemasa; Kurisaka, Kenichi; Takano, Kazuya; Kikuchi, Shin; Kondo, Toshiki; Umeda, Ryota; Shirakura, Shota*; Hayashi, Masaaki*
Proceedings of 8th International Conference on New Energy and Future Energy Systems (NEFES 2023) (Internet), p.27 - 34, 2023/00
Times Cited Count:0 Percentile:0.00(Green & Sustainable Science & Technology)This project studies investigation on safety design guideline and risk assessment technology for sodium-cooled fast reactor with the molten-salt heat storage system, development of evaluation method for heat transferring performance between sodium and molten-salt and improvement of the performance, and evaluation of chemical reaction characteristic between sodium and molten-salt and improvement of its safety. The project overview is presented in this report.
Sato, Rika*; Nishi, Tsuyoshi*; Ota, Hiromichi*; Hayashi, Hirokazu; Sugawara, Takanori; Nishihara, Kenji
Dai-43-Kai Nihon Netsu Bussei Shimpojiumu Koen Rombunshu (CD-ROM), 3 Pages, 2022/10
no abstracts in English
Kanamura, Shohei*; Takahashi, Yuya*; Omori, Takashi*; Nohira, Toshiyuki*; Sakamura, Yoshiharu*; Matsumura, Tatsuro
Denki Kagaku, 88(3), p.289 - 290, 2020/09
no abstracts in English
Sagayama, Yutaka; Ando, Masato
Nihon Genshiryoku Gakkai-Shi ATOMO , 60(3), p.162 - 167, 2018/03
, 60(3), p.162 - 167, 2018/03
The Generation IV international Forum (GIF) has led international collaborative efforts to develop six next generation nuclear energy systems, such as Sodium-cooled Fast Reactor (SFR), Lead-cooled Fast Reactor (LFR), Gas-cooled Fast Reactor (GFR), Molten Salt Reactor (MSR), Supercritical Water-cooled Reactor (SCWR), and Very High Temperature Reactor (VHTR), which have superior characteristics for the Safety and Reliability, Economics, Sustainability, Proliferation Resistance and Physical Protection. Some systems are already in the Demonstration Phase and the commercialization of the system in 2030s, which is the target of GIF, comes into sight.
Ogawa, Toru; Minato, Kazuo; Okamoto, Yoshihiro; Nishihara, Kenji
Journal of Nuclear Materials, 360(1), p.12 - 15, 2007/01
Times Cited Count:15 Percentile:68.94(Materials Science, Multidisciplinary)The actinide management has become a key issue in nuclear energy due to increasing proliferation concern and long-term environmental perception. The better way of waste management will be made by system symbiosis: a combination of light-water reactor and fast reactor and/or accelerator-driven transmutation system should be sought. The new recycling technology should be able to achieve good economy with smaller plants, which can process fuels from different types of reactors on a common technical basis. Pyroprocess with the use of molten salts is regarded as the strong candidate for such recycling technology. In JAEA, the first laboratory for the high temperature chemistry of transuranium elements, mainly Am and Cm, has been established. The fundamental data on the molten-salt chemistry of transuranium oxides and nitrides will be combined with the computer code for predicting the molten-salts electrolytic processes.
Shirai, Osamu*; Yamana, Hajimu*; Arai, Yasuo
Journal of Alloys and Compounds, 408-412, p.1267 - 1273, 2006/02
Times Cited Count:43 Percentile:85.00(Chemistry, Physical)no abstracts in English
 with alkali chlorides
with alkali chloridesOkamoto, Yoshihiro; Yaita, Tsuyoshi; Minato, Kazuo
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 73(8), p.745 - 747, 2005/08
Times Cited Count:0 Percentile:0.00(Electrochemistry)The structural change of molten PbCl by mixing with alkali chlorides was investigated by Pb L
by mixing with alkali chlorides was investigated by Pb L -edge XAFS measurements. By the mixing, the nearest neighbor Pb-Cl distance and its coordination number increased, while the Debye-Waller factor and the 3rd cumulant decreased. It is concluded that the coordination structure (PbCl
-edge XAFS measurements. By the mixing, the nearest neighbor Pb-Cl distance and its coordination number increased, while the Debye-Waller factor and the 3rd cumulant decreased. It is concluded that the coordination structure (PbCl )
) is enhanced by the mixing with alkali chlorides.
is enhanced by the mixing with alkali chlorides.
 in LiCl-KCl eutectic melt
in LiCl-KCl eutectic meltOkamoto, Yoshihiro; Yaita, Tsuyoshi; Minato, Kazuo
Journal of Molecular Structure, 749(1-3), p.70 - 73, 2005/07
Times Cited Count:3 Percentile:7.17(Chemistry, Physical)The local structures of molten BiCl and its mixtures in LiCl-KCl eutectic melt were investigated by X-ray absorption fine structure (XAFS) technique. The 1st Bi-Cl correlation in molten pure BiCl
and its mixtures in LiCl-KCl eutectic melt were investigated by X-ray absorption fine structure (XAFS) technique. The 1st Bi-Cl correlation in molten pure BiCl shows covalent nature, since the distance was almost the same as sum of covalent radii of Bi and Cl and the coordination number was almost 3. The similar property was observed also in the mixture of 75% BiCl
shows covalent nature, since the distance was almost the same as sum of covalent radii of Bi and Cl and the coordination number was almost 3. The similar property was observed also in the mixture of 75% BiCl with LiCl-KCl eutectic melt. Drastic change was detected in 25% BiCl
with LiCl-KCl eutectic melt. Drastic change was detected in 25% BiCl mixture melt. The 1st Bi-Cl distance was sum of ionic radii in molten 25% BiCl
mixture melt. The 1st Bi-Cl distance was sum of ionic radii in molten 25% BiCl melt. The results suggest that BiCl
melt. The results suggest that BiCl changes from molecular liquid to ionic by mixing with alkali chlorides.
changes from molecular liquid to ionic by mixing with alkali chlorides.

Okamoto, Yoshihiro; Iwadate, Yasuhiko*; Fukushima, Kazuko*; Matsuura, Haruaki*; Minato, Kazuo
Journal of Physics and Chemistry of Solids, 66(2-4), p.452 - 455, 2005/02
Times Cited Count:6 Percentile:29.10(Chemistry, Multidisciplinary)The structure of molten PbCl was investigated by using X-ray diffrtaction(XRD) and X-ray absorption fine structure(XAFS) analysis techniques. From the fourier transformation analysis if the XRD data, the nearest Pb
was investigated by using X-ray diffrtaction(XRD) and X-ray absorption fine structure(XAFS) analysis techniques. From the fourier transformation analysis if the XRD data, the nearest Pb -Cl
-Cl interaction consists of two kinds of interactions; rigid 4-fold tetrahedron and 2-3 loose coordination. The XAFS result shows a local structure is the 4-fold coordination in the melt.
interaction consists of two kinds of interactions; rigid 4-fold tetrahedron and 2-3 loose coordination. The XAFS result shows a local structure is the 4-fold coordination in the melt.
Okamoto, Yoshihiro; Madden, P. A.*
Journal of Physics and Chemistry of Solids, 66(1), p.448 - 451, 2005/01
The local structure of molten LaCl has been characterized by the octahedral coordination (LaCl
has been characterized by the octahedral coordination (LaCl )
) . It is based on X-ray diffraction (XRD) and Raman spectroscopy results. On the other hand, a different structural image was proposed in the neutron diffraction (ND) and the molecular dynamics (MD) studies. These ND and MD works concluded that a coordination behavior changes with cation size in molten rare earth trichlorides. Thus, they reported the coordination number of Cl
. It is based on X-ray diffraction (XRD) and Raman spectroscopy results. On the other hand, a different structural image was proposed in the neutron diffraction (ND) and the molecular dynamics (MD) studies. These ND and MD works concluded that a coordination behavior changes with cation size in molten rare earth trichlorides. Thus, they reported the coordination number of Cl ions around La
ions around La ion is 8.2 in the ND work and 7.9 in the MD work. In the present work, XRD measurements of molten LaCl
ion is 8.2 in the ND work and 7.9 in the MD work. In the present work, XRD measurements of molten LaCl were performed to investigate the local structure. The coordination number of the 1st La
were performed to investigate the local structure. The coordination number of the 1st La -Cl
-Cl pair in molten LaCl
pair in molten LaCl was 7.1 from analysis of the correlation function G(r). The structural change by increasing anion size was examined by the XRD measurement of molten LaBr
was 7.1 from analysis of the correlation function G(r). The structural change by increasing anion size was examined by the XRD measurement of molten LaBr . The XRD data were nicely reproduced by the MD simulations with polarizable ionic model.
. The XRD data were nicely reproduced by the MD simulations with polarizable ionic model.
 from molten salts
from molten saltsAbe, Hideki*; Yoshii, Kenji; Nishida, Kenji*; Imai, Motoharu*; Kitazawa, Hideaki*
Journal of Physics and Chemistry of Solids, 66(1), p.406 - 409, 2005/01
no abstracts in English
 systems
systemsOkamoto, Yoshihiro; Shiwaku, Hideaki; Yaita, Tsuyoshi; Suzuki, Shinichi; Minato, Kazuo; Tanida, Hajime*
Zeitschrift f r Naturforschung, A, 59a(11), p.819 - 824, 2004/11
r Naturforschung, A, 59a(11), p.819 - 824, 2004/11
The local structure of molten CdCl and CdCl
and CdCl -KCl mixture was investigated by high-temperature XAFS technique. The nearest Cd-Cl distance decreases by melting. Similarly, the coordination number decreases from 6 to 4. It suggests that there is (CdCl
-KCl mixture was investigated by high-temperature XAFS technique. The nearest Cd-Cl distance decreases by melting. Similarly, the coordination number decreases from 6 to 4. It suggests that there is (CdCl )
) tetrahedral complex ion in the melt. It is concluded that the local structure is kept also in the mixture melt, since the same XAFS result as the pure melt was obtained.
tetrahedral complex ion in the melt. It is concluded that the local structure is kept also in the mixture melt, since the same XAFS result as the pure melt was obtained.
Okamoto, Yoshihiro
Nuclear Instruments and Methods in Physics Research A, 526(3), p.572 - 583, 2004/07
Times Cited Count:32 Percentile:85.79(Instruments & Instrumentation)The XAFS functions for highly-disordered materials such as molten salts were simulated by using output data of molecular dynamics calculation. The atomic configuration data obtained from the MD results was directly used as input data in the XAFS simulation code FEFF8. It was assumed that Debye-Waller factor and anharmonic vibration effect could be substituted by an accumulation of the FEFF8 calculations using many atomic configurations. The experimental XAFS functions for platinum, solid and molten salt systems were nicely reproduced by the FEFF8 calculations with 10000-50000 atomic configurations.
Okamoto, Yoshihiro; Minato, Kazuo
JAERI-Conf 2004-008, 228 Pages, 2004/04
Applications of molten salts technology to separation and synthesis of materials have a potential to give us a civilized life, for example aluminium refinement. Recently, much attention is given to the pyrochemical reprocessing of spent nuclear fuels in the molten salt research field. On the other hand, computer simulation technique is expected to play an important role for supporting experimental works and predicting unknown physical properties in the molten salts application studies. Research group for Actinides Science, Department of Materials Science, Japan Atomic Energy Research Institute(JAERI), together with Reprocessing and Recycle Technology Division, Atomic Energy Society of Japan, organized the 3rd Workshop on Molten Salts Technology and Computer Simulation at Tokai Research Establishment, JAERI on December 16, 2003. Many molten salts researchers in Japan participated in the workshop and many useful presentations and discussions were performed.

Okamoto, Yoshihiro; Yaita, Tsuyoshi; Minato, Kazuo
Journal of Non-Crystalline Solids, 333(2), p.182 - 186, 2004/02
Times Cited Count:12 Percentile:67.86(Materials Science, Ceramics)The local structure of solid and molten SrCl was investigated by X-ray absorption fine structure(XAFS) technique. A superionic conduction state was observed below the melting point. The XAFS data at molten state was obtained at 1000
was investigated by X-ray absorption fine structure(XAFS) technique. A superionic conduction state was observed below the melting point. The XAFS data at molten state was obtained at 1000 C. According to the curve fitting analysis, the nearest Sr
C. According to the curve fitting analysis, the nearest Sr -Cl
-Cl distance and the coordination number are 2.99
distance and the coordination number are 2.99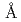 and 6.6 in molten state. XAFS functions calculated from molecular dynamics simulation results were copmpared with the experimental XAFS data.
and 6.6 in molten state. XAFS functions calculated from molecular dynamics simulation results were copmpared with the experimental XAFS data.