Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Takahashi, Rieko*; Taniguchi, Naoki
Zairyo To Kankyo, 73(6), p.153 - 163, 2024/06
Carbon steel is one of the candidate materials for overpacks in geological disposal of high-level radioactive waste, and is known to susceptible to stress corrosion cracking(SCC) depending on the condition in carbonate environment. In order to understand the influence of temperature on the SCC susceptibility of carbon steel, slow strain rate test (SSRT) of rolled steel were performed in NaHCO aqueous solution with varying temperature in the range of 303-393K for conditions of 0.1-0.5 mol/dm
aqueous solution with varying temperature in the range of 303-393K for conditions of 0.1-0.5 mol/dm , which is assumed to be the upper limit of carbonate concentration in groundwater in a geological disposal environment. As the results, no obvious influence of temperature on mechanical properties such as fracture strain ratio and reduction area ratio were observed, but SCC susceptibility based on SCC fracture ratio increased at relatively low temperatures of 303K and 323K. It was suggested that the reason for the higher SCC sensitivity at lower temperatures was due to slower repassivation at lower temperatures. Regarding the type of SCC, intergranular SCC was dominant at low temperatures and tended to transition to intergranular SCC at higher temperatures. Transgranular SCC tended to be observed at lower potentials than those at which intergranular SCC was observed.
, which is assumed to be the upper limit of carbonate concentration in groundwater in a geological disposal environment. As the results, no obvious influence of temperature on mechanical properties such as fracture strain ratio and reduction area ratio were observed, but SCC susceptibility based on SCC fracture ratio increased at relatively low temperatures of 303K and 323K. It was suggested that the reason for the higher SCC sensitivity at lower temperatures was due to slower repassivation at lower temperatures. Regarding the type of SCC, intergranular SCC was dominant at low temperatures and tended to transition to intergranular SCC at higher temperatures. Transgranular SCC tended to be observed at lower potentials than those at which intergranular SCC was observed.
Sakasegawa, Hideo; Nomura, Mitsuo; Sawayama, Kengo; Nakayama, Takuya; Yaita, Yumi*; Yonekawa, Hitoshi*; Kobayashi, Noboru*; Arima, Tatsumi*; Hiyama, Toshiaki*; Murata, Eiichi*
Progress in Nuclear Energy, 153, p.104396_1 - 104396_9, 2022/11
Times Cited Count:1 Percentile:12.98(Nuclear Science & Technology)When dismantling centrifuges in uranium-enrichment facilities, decontamination techniques must be developed to remove uranium-contaminated surfaces of dismantled parts selectively. Dismantled uranium-contaminated parts can be disposed of as nonradioactive wastes or recycled after decontamination appropriate for clearance. previously, we developed a liquid decontamination technique using acidic electrolyzed water to remove uranium-contaminated surfaces. However, further developments are still needed for its actual application. Dismantled parts have various uranium-contaminated surface features due to varied operational conditions, inhomogeneous decontamination using iodine heptafluoride gas, and changes in long-term storage conditions after dismantling. Here, we performed liquid decontamination on specimens with varying uranium-contaminated surfaces cut from a centrifuge made of low-carbon steel. From the results, the liquid decontamination can effectively remove the uranium-contaminated surfaces, and radioactive concentrations fell below the target value within twenty minutes. Although the required time should also depend on dismantled parts' sizes and shapes in their actual application, we demonstrated that it could be an effective decontamination technique for uranium-contaminated steels of dismantled centrifuges.
Otani, Kyohei; Ueno, Fumiyoshi; Kato, Chiaki
Zairyo To Kankyo, 71(2), p.40 - 45, 2022/02
The purpose of this study is to investigate the effect of oxygen concentration in the air on the corrosion rate of carbon steel in an air/solution alternating environment in the low oxygen concentration range and to clarify the corrosion rate and corrosion mechanism of carbon steel depending on the oxygen concentration in air by the mass change of specimens before and after the corrosion test and observing the iron rust layer formed on the surface of carbon steel. The corrosion rate increases with increasing oxygen concentration in the air, and the gradient of the corrosion rate decreases gradually. The maximum erosion depth increased with increasing oxygen concentration except for the case of 1% oxygen concentration, however, the maximum erosion depth for 1% oxygen concentration was larger than that for 5% air oxygen concentration.
Igarashi, Takahiro; Komatsu, Atsushi; Kato, Chiaki; Sakairi, Masatoshi*
Tetsu To Hagane, 107(12), p.998 - 1003, 2021/12
Times Cited Count:1 Percentile:4.52(Metallurgy & Metallurgical Engineering)In order to clarify the effect of environmental factors on the amount of atmospheric corrosion of steel, novel model for predicting the reduction of atmospheric corrosion considering relative humidity and rain falls was developed. We conducted a one-year calculation simulation of atmospheric corrosion in Miyakojima City, Choshi City, and Tsukuba City using the developed model. Corrosion weight loss by the simulation could reproduce the measured value well. Corrosion weight loss at each point was greatly affected by the amount of flying sea salt, relative humidity, and rain falls.
Sato, Tomonori; Komatsu, Atsushi; Nakano, Junichi*; Yamamoto, Masahiro*
Zairyo To Kankyo, 70(12), p.457 - 461, 2021/12
The Primary Containment Vessel (PCV) in Fukushima Daiichi Nuclear Power Plants (1F) have been exposed to a corrosive environment containing seawater components under irradiation condition. In such conditions, the hydrogen peroxide (H O
O ) is generated by water radiolysis as an oxidant in addition to dissolved oxygen. The H
) is generated by water radiolysis as an oxidant in addition to dissolved oxygen. The H O
O concentration is able to be estimated by radiolysis calculations. The corrosion tests under gamma-ray irradiation was performed. As the results, it was confirmed that the N
concentration is able to be estimated by radiolysis calculations. The corrosion tests under gamma-ray irradiation was performed. As the results, it was confirmed that the N gas purging performed in the PCV is effective in inhibiting the corrosion of carbon steel under irradiation conditions. The weight loss of carbon steel obtained in the corrosion test under gamma-ray irradiations, are evaluated using calculated O
gas purging performed in the PCV is effective in inhibiting the corrosion of carbon steel under irradiation conditions. The weight loss of carbon steel obtained in the corrosion test under gamma-ray irradiations, are evaluated using calculated O and H
and H O
O concentrations obtained by radiolysis calculations. As a results, it is confirmed that the corrosion rate of carbon steel under irradiation is determined by the sum of diffusion limiting currents of O
concentrations obtained by radiolysis calculations. As a results, it is confirmed that the corrosion rate of carbon steel under irradiation is determined by the sum of diffusion limiting currents of O and H
and H O
O as well as that of carbon steel in the O
as well as that of carbon steel in the O and H
and H O
O coexistent conditions under non-irradiation.
coexistent conditions under non-irradiation.
Otani, Kyohei; Kato, Chiaki
Zairyo To Kankyo, 70(12), p.480 - 486, 2021/12
This is a comprehensive paper of the corrosion of carbon steel in air/solution alternating condition. From cross-sectional observation and analysis of the iron rust layer formed on the surface of carbon steel in the alternating condition, it was found that a multilayered iron rust layer composed of red rust layer ( -FeOOH), rust crust layer (Fe
-FeOOH), rust crust layer (Fe O
O ), inner crystal (Fe
), inner crystal (Fe O
O ), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
Igarashi, Takahiro; Komatsu, Atsushi; Kato, Chiaki; Sakairi, Masatoshi*
Bosei Kanri, 65(10), p.365 - 370, 2021/10
We have developed a new atmospheric simulation model considering important environmental factors such as airborne sea salt, temperature, relative humidity, and rainfall. The developed model was verified by comparing predicted values by the simulation and measured data for the weight loss by atmospheric corrosion. In addition, atmospheric corrosion simulations under open and sheltered exposure condition were conducted, and it was confirmed that the air corrosion weight loss was strongly suppressed by the surface cleaning effect due to rainfall.
Otani, Kyohei; Tsukada, Takashi; Ueno, Fumiyoshi; Kato, Chiaki
Zairyo To Kankyo, 69(9), p.246 - 252, 2020/09
The purpose of this study was to investigate the effect of artificial sea water concentration on the corrosion rate of carbon steel under air/solution alternating condition, and to clarify the corrosion mechanism of carbon steel that changes with artificial seawater concentration. Mass measurements showed that the corrosion rate of carbon steel in the alternating condition accelerates with increasing concentration in the concentration region between deionized water to 200 times diluted artificial seawater (ASW), and the corrosion rate decreases with increasing concentration in the concentration region between 20 times diluted ASW to undiluted ASW. It can be considered that the reason why the carbon steel corrosion was suppressed in highly concentrated artificial seawater would Mg ions and Ca ions in the artificial seawater precipitate and cover on the surface due to the increase in pH near the surface by oxygen reduction reaction.
Omori, Atsushi*; Akiyama, Eiji*; Abe, Hiroshi*; Hata, Kuniki; Sato, Tomonori; Kaji, Yoshiyuki; Inoue, Hiroyuki*; Taguchi, Mitsumasa*; Seito, Hajime*; Tada, Eiji*; et al.
Zairyo To Kankyo, 69(4), p.107 - 111, 2020/04
To evaluate the effect of oxidants, which are formed by radiolysis of water under gamma ray irradiation, on the corrosion of a carbon steel in humid environment, ozone was introduced as a model oxidant in to humidity-controlled air at 50 C in a thermo-hygrostat chamber. Corrosion monitoring was performed by using an Atmospheric Corrosion Monitor-type (ACM) sensor consisting of a carbon steel anode and an Ag cathode. The output current of the ACM sensor was increased with the increase in relative humidity and it was obviously increased with the increase in the introduced ozone concentration at each relative humidity. The results indicate that ozone accelerates the corrosion of the carbon steel. The effect of ozone on the corrosion acceleration is attributed to the fast reduction reaction and fast dissolution reaction in to water compared to that of oxygen.
C in a thermo-hygrostat chamber. Corrosion monitoring was performed by using an Atmospheric Corrosion Monitor-type (ACM) sensor consisting of a carbon steel anode and an Ag cathode. The output current of the ACM sensor was increased with the increase in relative humidity and it was obviously increased with the increase in the introduced ozone concentration at each relative humidity. The results indicate that ozone accelerates the corrosion of the carbon steel. The effect of ozone on the corrosion acceleration is attributed to the fast reduction reaction and fast dissolution reaction in to water compared to that of oxygen.
Islam, M. S.*; Otani, Kyohei; Sakairi, Masatoshi*
ISIJ International, 58(9), p.1616 - 1622, 2018/09
Times Cited Count:7 Percentile:30.67(Metallurgy & Metallurgical Engineering)To elucidate the role of Zn on corrosion of coated steel, the effects of metal cations on the corrosion of carbon steel in the concentrated Cl
on corrosion of coated steel, the effects of metal cations on the corrosion of carbon steel in the concentrated Cl aqueous solutions were studied by immersion tests, surface analysis and electrochemical tests. Among the examined metal cations, Zn
aqueous solutions were studied by immersion tests, surface analysis and electrochemical tests. Among the examined metal cations, Zn showed the significant effect on corrosion inhibition of carbon steel in the Cl
showed the significant effect on corrosion inhibition of carbon steel in the Cl aqueous solution at high concentration. XPS analysis results elucidated that Zn
aqueous solution at high concentration. XPS analysis results elucidated that Zn can remain on the steel surface after immersed in the solutions with Zn
can remain on the steel surface after immersed in the solutions with Zn . EIS measurements showed higher impedance in the solution with Zn
. EIS measurements showed higher impedance in the solution with Zn than other solutions, and the results suggested that Zn
than other solutions, and the results suggested that Zn reduced the defect points in the thin oxide film by forming a metal cation layer. Based on the experimental results, Zn
reduced the defect points in the thin oxide film by forming a metal cation layer. Based on the experimental results, Zn may form a layer on the oxide film that protects the Cl
may form a layer on the oxide film that protects the Cl attack in the solution. The findings demonstrated that the formation of Zn layer on the oxide film is one of the main reason for showing high and longtime corrosion resistance of Zn coated steel substrate.
attack in the solution. The findings demonstrated that the formation of Zn layer on the oxide film is one of the main reason for showing high and longtime corrosion resistance of Zn coated steel substrate.
 O in Fe
O in Fe O
O film on Fe formed in an NaOH solution containing oxidants
film on Fe formed in an NaOH solution containing oxidantsHaruna, Takumi*; Miyataki, Yuki*; Hirohata, Yohei*; Shibata, Toshio*; Taniguchi, Naoki; Tachikawa, Hirokazu*
Zairyo To Kankyo, 67(9), p.375 - 380, 2018/09
This research aimed to confirm the formation of Fe O
O film on Fe immersed in aqueous 45 mass% NaOH solution containing some oxidants at the boiling temperature, to recognize the optimum immersion time for the formation of thick and protective film, and to reveal the absorption behavior of D
film on Fe immersed in aqueous 45 mass% NaOH solution containing some oxidants at the boiling temperature, to recognize the optimum immersion time for the formation of thick and protective film, and to reveal the absorption behavior of D O in the Fe
O in the Fe O
O film at room temperature. The results were obtained as follows. It was confirmed that Fe
film at room temperature. The results were obtained as follows. It was confirmed that Fe O
O film was formed on Fe immersed in the NaOH solution for a time more than 0.6 ks, and the film thickness increased parabolically with an increase in the immersion time. D
film was formed on Fe immersed in the NaOH solution for a time more than 0.6 ks, and the film thickness increased parabolically with an increase in the immersion time. D O absorption test was carried out to the films formed in the NaOH solution for immersion times of 1.2 and 3.6 ks. An amount of D
O absorption test was carried out to the films formed in the NaOH solution for immersion times of 1.2 and 3.6 ks. An amount of D O absorbed into the film increased with an increase in an absorption time up to 1000 ks, and an absorption time more than 1000 ks made an amount of D
O absorbed into the film increased with an increase in an absorption time up to 1000 ks, and an absorption time more than 1000 ks made an amount of D O constant. The constant amount of D
O constant. The constant amount of D O was larger for the film formed on Fe immersed in the NaOH solution for 3.6 ks than that for 1.2 ks. The transient of the amount of D
O was larger for the film formed on Fe immersed in the NaOH solution for 3.6 ks than that for 1.2 ks. The transient of the amount of D O absorbed into the film was analyzed on the basis of Fick's law for diffusion, and diffusion coefficients of D
O absorbed into the film was analyzed on the basis of Fick's law for diffusion, and diffusion coefficients of D O were obtained to be 5.1
O were obtained to be 5.1 10
10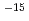 cm
cm s
s and 9.9
and 9.9 10
10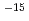 cm
cm s
s for the films formed for 1.2 and 3.6 ks, respectively. Therefore it was estimated that the diffusion coefficient of the Fe
for the films formed for 1.2 and 3.6 ks, respectively. Therefore it was estimated that the diffusion coefficient of the Fe O
O film was in the region from 5.1
film was in the region from 5.1 10
10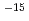 cm
cm s
s to 9.9
to 9.9 10
10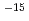 cm
cm s
s .
.
 -ray irradiated neutral water condition
-ray irradiated neutral water conditionYamamoto, Masahiro; Komatsu, Atsushi; Sato, Tomonori; Nakano, Junichi; Ueno, Fumiyoshi
Proceedings of 17th Asian Pacific Corrosion Control Conference (APCCC-17) (USB Flash Drive), 8 Pages, 2016/01
In Fukushima-Daiichi Nuclear Power Station, decommissioning procedures are continuing more than 30 years until fuel debris removal. It is important to keep soundness of primary container vessel (PCV), made of carbon steel, during these procedures. Corrosion of carbon steel is well-known to be controlled by cathodic reaction, in usual, oxygen reduction reaction. Corrosion of carbon steel could be mitigated by nitrogen injection procedure. However, a lot of radioactive materials exist in cooling water, an effect of radiolysis product on corrosion is an important problem. Clarifying an irradiation effect for corrosion of carbon steel, corrosion test was conducted in 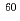 Co
Co  -ray irradiated condition. Electrochemical measurements were conducted to determine cathodic current density of samples. Corrosion rates of carbon steel decrease with time in both
-ray irradiated condition. Electrochemical measurements were conducted to determine cathodic current density of samples. Corrosion rates of carbon steel decrease with time in both  -ray irradiated and non-irradiated conditions. Measured values of cathodic current density gradually decreased with time and then stayed at constant value.
-ray irradiated and non-irradiated conditions. Measured values of cathodic current density gradually decreased with time and then stayed at constant value.
 -ray irradiation
-ray irradiationMotooka, Takafumi; Ueno, Fumiyoshi
Zairyo To Kankyo, 64(6), p.220 - 223, 2015/06
Corrosion behavior of carbon steel in chloride aqueous solutions under a low dose rate was investigated by corrosion test using chloride aqueous solutions with different chloride concentration at a dose rate of 500 Gy/h. The corrosion rate of carbon steel increased by the irradiation, and the corrosion rate had the maximum value at a certain chloride concentration. The oxidants produced by radiolysis of chloride aqueous solution enhanced the corrosion of carbon steel. The main oxidants were oxygen and hydrogen peroxide, and the diffusion process of oxidants controlled the corrosion of carbon steel under irradiation. There was a positive correlation between the dependence of corrosion rate and chloride concentration and the dependence of oxidant concentration and chloride concentration.
Mitsui, Seiichiro
Koeki Zaidan Hojin Tottoriken Kyoiku Bunka Zaidanhen 2014 "Yoshida Nakamichi Iseki" Tottoriken Kyoiku Iinkai, p.221 - 230, 2015/03
An ancient socketed iron axe was excavated from Yoshida Nakamichi site in Tottori City, Tottori Prefecture. To understand reasons of corrosion state of the axe, we studied relationship between burial environment and corrosion. As environmental conditions, we investigated groundwater chemistry and corrosion rate with iron probe monitor, etc. As for corrosion state, we analysed corrosion depths with a X-ray CT and corrosion products with a portable XRD/XRF. As results, we found that the redox potential and dissolved oxygen level as environmental conditions were very low, and that the maximum corrosion rate (2 10
10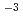 mm/y) evaluated from measured corrosion depths was smaller than the probe corrosion rate (5
mm/y) evaluated from measured corrosion depths was smaller than the probe corrosion rate (5 10
10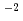 mm/y) by two orders of magnitude and identified siderite (FeCO
mm/y) by two orders of magnitude and identified siderite (FeCO ) as a corrosion product. The results suggested that the siderite precipitated on the surface of the iron sword inhibited corrosion reaction.
) as a corrosion product. The results suggested that the siderite precipitated on the surface of the iron sword inhibited corrosion reaction.
Taniguchi, Naoki; Kawakami, Susumu;
JNC TN8400 2001-025, 27 Pages, 2002/03
It is essential to understand the corrosion type of carbon steel under the repository conditions for the lifetime assessment of carbon steel overpack used for geological isolation of high-level radioactive waste. According to the previous study, carbon steel is hard to passivate in buffer material assuming a chemical condition range of groundwater in Japan. However, concrete support will be constructed around the overpack in the case of repository in the soft rock system and groundwater having a higher pH may infiltrate to buffer material. There is a possibility that the corrosion type of carbon steel will be influenced by the rise of the pH in groundwater. In this study, anodic polarization experiments were performed to understand the passivation condition of carbon steel in buffer material saturated with water contacted with concrete. An ordinary concrete and a low-alkalinity concrete were used in the experiment. The results of the experiments showed that the carbon steel can passivate under the condition that water having pH  13 infiltrate to the buffer material assuming present property of buffer material. If the low-alkalinity concrete is selected as the support material, passivation can not occur on carbon steel overpack. The effect of the factors of buffer material such as dry density and mixing ratio of sand on the passivation of carbon steel was also studied. The results of the study showed that the present property of buffer material is enough to prevent passivation of carbon steel.
13 infiltrate to the buffer material assuming present property of buffer material. If the low-alkalinity concrete is selected as the support material, passivation can not occur on carbon steel overpack. The effect of the factors of buffer material such as dry density and mixing ratio of sand on the passivation of carbon steel was also studied. The results of the study showed that the present property of buffer material is enough to prevent passivation of carbon steel.
Inoue, Kazuko*; Nakamura, Hiroshi*; Horikawa, Takeshi*; Kawashima, Hisakazu*; Tsujikami, Tetsuya*; Minakawa, Nobuaki
Nihon Zairyo Gakkai Dai-50-Ki Gakujutsu Koenkai Koen Rombunshu, p.415 - 416, 2001/05
no abstracts in English
Taniguchi, Naoki; Kawasaki, Manabu*; Fujiwara, Kazuo*
JNC TN8400 2001-011, 62 Pages, 2001/03
The corrosion of metallic materials used in natural environment are sometimes affected by microbial action. It is apprehended that microorganism living in deep underground or brought from ground surface during excavation makes an impact on overpack material for geological disposal of high-level radioactive waste. Sulfate reducing bacteria (SRB) is known to be one of the most representative microorganism which affects the corrosion of metals. In this study, the behavior of growth of SRB was investigated at first under the presence of bentonite as a main component of buffer material which encloses the overpack. The results of the tests showed that the population of SRB after the culture in synthetic sea water mixed with bentonite decreased with increasing the ratio of bentonite/solution. SRB was hardly grown in medium whose bentonite/solution ratio exceeded 1000g/l. As a conservative case, the effects of sulfide on the corrosion of overpack materials were also studied assuming high activity of SRB. Carbon steel, copper and titanium specimens were immersed in synthetic sea water purging 0.1MPa H S gas and the corrosion behavior was compared with the results in N
S gas and the corrosion behavior was compared with the results in N gas purging environment. Obvious effect of sulfide on the corrosion of carbon steel was not observed, but the corrosion rates of copper specimens were accelerated several hundred times by purging H
gas purging environment. Obvious effect of sulfide on the corrosion of carbon steel was not observed, but the corrosion rates of copper specimens were accelerated several hundred times by purging H S gas. The absorption of hydrogen into titanium specimens was not affected by purging H
S gas. The absorption of hydrogen into titanium specimens was not affected by purging H S gas, but the difference of hydrogen absorption between pure titanium and titanium alloy containing 0.06%-Pd was observed.
S gas, but the difference of hydrogen absorption between pure titanium and titanium alloy containing 0.06%-Pd was observed.
Taniguchi, Naoki; ; Kawasaki, Manabu*; Masugata, Tsuyoshi*
JNC TN8400 2001-001, 56 Pages, 2000/12
It is necessary to clear the effects of corrosion products on the corrosion life time of carbon steel overpack for geological isolation of high-level radioactive waste(HLW). Especially, it is important to understand the effects of magnetite because magnetite as a simulated corrosion product is reported to accelerate the corrosion rate of carbon steel. In this study, corrosion tests to reproduce the acceleration of corrosion due to magnetite was performed and the mechanism of the acceleration was investigated to evaluate the effects of magnetite as a corrosion product. Based on the results of experiments, following conclusions are obtained ; (1)Magnetite powder accelerates the corrosion rate of carbon steel. The main reaction of corrosion under the presence of magnetite is the reduction of Fe(III) in magnetite to Fe(II), but the reaction of hydrogen generation is also accelerated. The contribution of hydrogen generation reaction was estimated to be about 30% in the total corrosion reaction based on the experimental result of immersion test under the presence of magnetite. (2)Actual corrosion products containing magnetite generated by the corrosion of carbon steel protect the metal from the propagation of corrosion. The corrosion depth of carbon steel overpack due to magnetite was estimated to be about 1 mm based on the results of experiments. Even if the effect of magnetite is taken into the assessment of corrosion lifetime of overpack, total corrosion depth in 1000 years is estimated to be 33 mm, which is smaller than the corrosion allowance of 40 mm described in the second progress report on research and development for the geological disposal of HLM/ in Japan. It was concluded that the effect of magnetite on the corrosion life time of carbon steel overpack is negligible.
; ; Ohno, Shuji;
JNC TN9400 2000-092, 247 Pages, 2000/08
Small-scale sodium pool combustion tests Run-F7-3 and Run-F8-1 were performed to investigate the corrosion of floor liner under high moisture condition. ln the both tests, which were performed using the 3m FRAT-1 vessel at the SAPFIRE facility, the sodium of 507deg-C was leaked on the carbon steel catch pan about for 25 minutes with the flow rate of around 25 kg/h. The air in the vessel was ventilated with the flow rate of 5m
FRAT-1 vessel at the SAPFIRE facility, the sodium of 507deg-C was leaked on the carbon steel catch pan about for 25 minutes with the flow rate of around 25 kg/h. The air in the vessel was ventilated with the flow rate of 5m /min containing the moisture of 25000-28000 vol.ppm. The duration of combustion was different in two tests by changing the starting time of argon gas injection into the vessel. As the results of post-test analysis such as observation of catch pan surface and chemical analysis of the deposits, it was confirmed that 'Na-Fe double oxidization type corrosion' was dominant in the both tests and that the catch pan and deposits were not under the condition leading to the occurrence of 'molten salt type corrosion.'
/min containing the moisture of 25000-28000 vol.ppm. The duration of combustion was different in two tests by changing the starting time of argon gas injection into the vessel. As the results of post-test analysis such as observation of catch pan surface and chemical analysis of the deposits, it was confirmed that 'Na-Fe double oxidization type corrosion' was dominant in the both tests and that the catch pan and deposits were not under the condition leading to the occurrence of 'molten salt type corrosion.'
Harada, Yuhei; Maruyama, Yu; Maeda, Akio*; Chino, Eiichi; Shibazaki, Hiroaki*; Kudo, Tamotsu; Hidaka, Akihide; Hashimoto, Kazuichiro; Sugimoto, Jun
Journal of Nuclear Science and Technology, 37(6), p.518 - 529, 2000/06
no abstracts in English