Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 based on experimental data and density functional theory calculation result
based on experimental data and density functional theory calculation resultWatanabe, Masashi; Nakamura, Hiroki; Suzuki, Kiichi; Machida, Masahiko; Kato, Masato
Journal of the American Ceramic Society, 105(3), p.2248 - 2257, 2022/03
Times Cited Count:1 Percentile:6.56(Materials Science, Ceramics)Properties of CeO were evaluated by DFT simulation to determine band gap, Frenkel defect formation energy and defect migration energy. Band gap and Frenkel defect formation energy were used to analyze defect equilibria. Oxygen partial pressure dependence of defect equilibria was evaluated based on oxygen potential experimental data and DFT calculation, and a Brouwer diagram was derived. The defect formation energies, including Frenkel defect, electron-hole pair and so on, were determined and used to evaluate the properties, including oxygen diffusion coefficients, electrical conduction, heat capacity and thermal conductivity. Mechanisms of various properties were discussed for a deeper understanding based on defect chemistry, and the relationship among properties were systematically described.
were evaluated by DFT simulation to determine band gap, Frenkel defect formation energy and defect migration energy. Band gap and Frenkel defect formation energy were used to analyze defect equilibria. Oxygen partial pressure dependence of defect equilibria was evaluated based on oxygen potential experimental data and DFT calculation, and a Brouwer diagram was derived. The defect formation energies, including Frenkel defect, electron-hole pair and so on, were determined and used to evaluate the properties, including oxygen diffusion coefficients, electrical conduction, heat capacity and thermal conductivity. Mechanisms of various properties were discussed for a deeper understanding based on defect chemistry, and the relationship among properties were systematically described.
 with Pu = 0.45 and 0.68 and related defect formation energy
with Pu = 0.45 and 0.68 and related defect formation energyHirooka, Shun; Matsumoto, Taku; Sunaoshi, Takeo*; Hino, Tetsushi*
Journal of Nuclear Materials, 558, p.153375_1 - 153375_8, 2022/01
Times Cited Count:2 Percentile:30.55(Materials Science, Multidisciplinary)no abstracts in English
Ikusawa, Yoshihisa; Maeda, Koji; Kato, Masato; Uno, Masayoshi*
Nuclear Technology, 199(1), p.83 - 95, 2017/07
Times Cited Count:4 Percentile:36.71(Nuclear Science & Technology)Based on thermal computation results obtained using an irradiation behavior analysis code, we have evaluated the effect of O/M ratio on fuel restructuring from the results of PIEs for the B14 irradiation test fuel, which was a mixed oxide fuel and was irradiated in the experimental reactor Joyo. The thermal computation results showed that fuel restructuring in the stoichiometric oxide fuel was accelerated, though the fuel temperature in the stoichiometric oxide fuel was evaluated as lower than that of the hypo-stoichiometric one. We explained this behavior as follows: first, the fuel temperature decreased due to the high thermal conductivity at stoichiometry; second, the pore migration velocity increased due to the increase in vapor pressure caused by the high vapor pressure of UO , which was derived from the high oxygen potential at stoichiometry. In addition, our results indicated that the central void diameter strongly depended on not only fuel temperature, but also vapor pressure.
, which was derived from the high oxygen potential at stoichiometry. In addition, our results indicated that the central void diameter strongly depended on not only fuel temperature, but also vapor pressure.

Kato, Masato; Watanabe, Masashi; Matsumoto, Taku; Hirooka, Shun; Akashi, Masatoshi
Journal of Nuclear Materials, 487, p.424 - 432, 2017/04
Times Cited Count:12 Percentile:75.2(Materials Science, Multidisciplinary)Oxygen potential of (U,Pu)O was evaluated based on defect chemistry using an updated experimental data set. The relationship between oxygen partial pressure and deviation
was evaluated based on defect chemistry using an updated experimental data set. The relationship between oxygen partial pressure and deviation  in (U,Pu)O
in (U,Pu)O was analyzed, and equilibrium constants of defect formation were determined as functions of Pu content and temperature. Brouwer's diagrams were constructed using the determined equilibrium constants, and a relational equation to determine O/M ratio was derived as functions of O/M ratio, Pu content and temperature. In addition, relationship between oxygen potential and oxygen diffusion coefficients were described.
was analyzed, and equilibrium constants of defect formation were determined as functions of Pu content and temperature. Brouwer's diagrams were constructed using the determined equilibrium constants, and a relational equation to determine O/M ratio was derived as functions of O/M ratio, Pu content and temperature. In addition, relationship between oxygen potential and oxygen diffusion coefficients were described.
 -CeO
-CeO
Hirooka, Shun; Murakami, Tatsutoshi; Nelson, A. T.*; McClellan, K. J.*
INL/EXT-14-33515, p.34 - 36, 2014/10
Collaborative study has been done on the properties of nuclear materials between the DOE and Japan and the oxygen potential of (U,Ce)O was measured in this year. Experimental measurements of the oxygen potential were conducted on Ce=20% and 30% composition rates simulating Pu content in advanced MOX fuel in JAEA by gas equilibrium method where oxygen partial pressure in the atmosphere was controlled with mixing dry/wet Ar/H
was measured in this year. Experimental measurements of the oxygen potential were conducted on Ce=20% and 30% composition rates simulating Pu content in advanced MOX fuel in JAEA by gas equilibrium method where oxygen partial pressure in the atmosphere was controlled with mixing dry/wet Ar/H gas. More than 100 data points were obtained in the O/M range of 1.945
gas. More than 100 data points were obtained in the O/M range of 1.945  2.000 at 1200
2.000 at 1200 C, 1400
C, 1400 C and 1600
C and 1600 C. The experimental results were analyzed by the defect analysis and analytical equations were obtained to calculate O/M as functions of temperature and oxygen potential. From the comparison with that of (U,Pu)O
C. The experimental results were analyzed by the defect analysis and analytical equations were obtained to calculate O/M as functions of temperature and oxygen potential. From the comparison with that of (U,Pu)O , applicability of the same defect chemistry and S-style curve are common. Also, it is revealed that (U,Ce)O
, applicability of the same defect chemistry and S-style curve are common. Also, it is revealed that (U,Ce)O requires evidently higher oxygen potential for the O/M.
requires evidently higher oxygen potential for the O/M.
 Pu
Pu O
O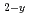 by EMF method
by EMF methodOtobe, Haruyoshi; Nakamura, Akio; Yamashita, Toshiyuki; Minato, Kazuo
Journal of Nuclear Materials, 344(1-3), p.219 - 222, 2005/09
Times Cited Count:3 Percentile:24.17(Materials Science, Multidisciplinary)Actinides-bearing zirconias are prominent candidate materials for various nuclear applications: targets for actinide transmutation, inert material fuels, radioactive waste forms, etc. In this study, the oxygen potential (g(O )) behavior of fluorite-type (F-type) Zr
)) behavior of fluorite-type (F-type) Zr Pu
Pu O
O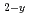 has been measured by EMF method using the zirconia oxygen sensor. It was found that the g(O
has been measured by EMF method using the zirconia oxygen sensor. It was found that the g(O ) values of F-type Zr
) values of F-type Zr Pu
Pu O
O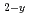 were about 150 kJ/mol higher than those of F-type PuO
were about 150 kJ/mol higher than those of F-type PuO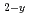 at the same oxygen-nonstoichiometric (O/M) values. The g(O
at the same oxygen-nonstoichiometric (O/M) values. The g(O ) values of F-type Zr
) values of F-type Zr Pu
Pu O
O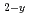 were 50 kJ/mol lower than those of pyrochlore Zr
were 50 kJ/mol lower than those of pyrochlore Zr Pu
Pu O
O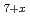 at the same O/M values. These results suggest that the g(O
at the same O/M values. These results suggest that the g(O ) behavior depends on the cation and anion ordering/disordering, in addition to the cation composition ratio (Zr/Pu).
) behavior depends on the cation and anion ordering/disordering, in addition to the cation composition ratio (Zr/Pu).
 solid solution with magnesium and europium oxides
solid solution with magnesium and europium oxidesFujino, Takeo*; Sato, Nobuaki*; Yamada, Kota*; Nakama, Shohei*; Fukuda, Kosaku; Serizawa, Hiroyuki; Shiratori, Tetsuo*
Journal of Nuclear Materials, 297(3), p.332 - 340, 2001/09
Times Cited Count:4 Percentile:33.39(Materials Science, Multidisciplinary)Oxygen potential of solid solution Mg Eu
Eu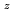 U
U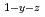 O
O was examined as a function of O/Metal ratio at 1000, 1100 and 1200
was examined as a function of O/Metal ratio at 1000, 1100 and 1200 C. The O/Metal ratio which gave the steepest change of the oxygen potential(GOM) was 1.995 for y=0.05, z=0.1 and y=0.05, z=0.05. The position decreased to 1.908 at higher Mg
C. The O/Metal ratio which gave the steepest change of the oxygen potential(GOM) was 1.995 for y=0.05, z=0.1 and y=0.05, z=0.05. The position decreased to 1.908 at higher Mg concentration of y=0.1, z=0.05. The GOM did not change with temperature in a range 1000-1200
concentration of y=0.1, z=0.05. The GOM did not change with temperature in a range 1000-1200 C. At GOM, a friction of 0.473 of total Mg
C. At GOM, a friction of 0.473 of total Mg occupy the interstitial position of the fluorite lattice.
occupy the interstitial position of the fluorite lattice.
Yamawaki, Michio*; Yamaguchi, Kenji*; Ono, Futaba*; Huang, J.*; Harada, Yuhei; Hidaka, Akihide; Sugimoto, Jun
JAERI-Tech 2000-015, p.38 - 0, 2000/03
no abstracts in English
*; Endo, Yasuichi; Yamaura, Takayuki; Matsui, Yoshinori; Niimi, Motoji; Hoshiya, Taiji; Kobiyama, M.*; Motohashi, Yoshinobu*
JAERI-Conf 99-006, p.343 - 348, 1999/08
no abstracts in English
 Gd
Gd O
O by solid state EMF measurements
by solid state EMF measurementsNakamura, Akio
Zeitschrift f r Physikalische Chemie, 207, p.223 - 243, 1998/00
r Physikalische Chemie, 207, p.223 - 243, 1998/00
no abstracts in English
Sato, Isamu*; *; ; Arima, Tatsumi*; ; Kajitani, Yukio
PNC TY9606 97-001, 117 Pages, 1997/07
no abstracts in English
Saito, Junichi; Hoshiya, Taiji; ;
JAERI-Tech 96-015, 58 Pages, 1996/03
no abstracts in English
Ugajin, Mitsuhiro; Nagasaki, Takanori*; Ito, Akinori
Journal of Nuclear Materials, 230(3), p.195 - 207, 1996/00
Times Cited Count:7 Percentile:54.39(Materials Science, Multidisciplinary)no abstracts in English
Ugajin, Mitsuhiro
JAERI-M 92-065, 113 Pages, 1992/05
no abstracts in English
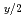 Y
Y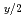 U
U O
O (x
(x 0,x=0,x
0,x=0,x 0)
0)Fujino, Takeo*; Yamashita, Toshiyuki; Ouchi, Kinji
Journal of Nuclear Materials, 183, p.46 - 56, 1991/00
Times Cited Count:9 Percentile:69.44(Materials Science, Multidisciplinary)no abstracts in English
 U
U O
O (x
(x 0,x
0,x 0) and rhombohedral Eu
0) and rhombohedral Eu UO
UO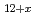 (x
(x 0)
0)Fujino, Takeo; Ouchi, Kinji; *; *; *
Journal of Nuclear Materials, 174, p.92 - 101, 1990/00
Times Cited Count:25 Percentile:89.06(Materials Science, Multidisciplinary)no abstracts in English
Journal of Nuclear Materials, 114(2-3), p.242 - 249, 1983/00
Times Cited Count:0 Percentile:0.02(Materials Science, Multidisciplinary)no abstracts in English
JAERI-M 8688, 44 Pages, 1980/02
no abstracts in English
 at high oxygen potential
at high oxygen potentialUgajin, Mitsuhiro; Shiba, Koreyuki
Journal of Nuclear Materials, 91(1), p.227 - 230, 1980/00
Times Cited Count:14 Percentile:94.62(Materials Science, Multidisciplinary)no abstracts in English

Murakami, Tatsutoshi; Kato, Masato; Nelson, A.*; McClellan, K.*
no journal, ,
no abstracts in English