Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O
 /U
/U O
O
 redox equilibrium in [emim]Tf
redox equilibrium in [emim]Tf N-based liquid electrolytes towards uranium redox-flow battery
N-based liquid electrolytes towards uranium redox-flow batteryTakao, Koichiro*; Ouchi, Kazuki; Komatsu, Atsushi; Kitatsuji, Yoshihiro; Watanabe, Masayuki
European Journal of Inorganic Chemistry, 27(14), p.e202300787_1 - e202300787_7, 2024/05
Times Cited Count:1 Percentile:0.00(Chemistry, Inorganic & Nuclear)Electrochemical behavior of U O
O
 /U
/U O
O
 in 1-ethyl-3-methylimidazolium bis(trifluoromethyl)sulfonylamide ([emim]Tf
in 1-ethyl-3-methylimidazolium bis(trifluoromethyl)sulfonylamide ([emim]Tf N) ionic liquid was studied to clarify what are required to attain its redox reversibility for utilizing depleted U as an electrode active material in a redox flow battery. As a result, reversibility of the U
N) ionic liquid was studied to clarify what are required to attain its redox reversibility for utilizing depleted U as an electrode active material in a redox flow battery. As a result, reversibility of the U O
O
 /U
/U O
O
 redox reaction was successfully achieved in use of a glassy carbon working electrode under presence of Cl
redox reaction was successfully achieved in use of a glassy carbon working electrode under presence of Cl in [emim]Tf
in [emim]Tf N. To improve diffusivity of solutes, [emim]Tf
N. To improve diffusivity of solutes, [emim]Tf N diluted with an auxiliary molecular solvent, N,N-dimethylformamide (DMF). We have succeeded in demonstrating a reversible redox reaction of [U
N diluted with an auxiliary molecular solvent, N,N-dimethylformamide (DMF). We have succeeded in demonstrating a reversible redox reaction of [U O
O Cl
Cl ]
] + e
+ e = [U
= [U O
O Cl
Cl ]
] in the 50:50 v/v [emim]Tf
in the 50:50 v/v [emim]Tf N-DMF liquid electrolyte containing Cl
N-DMF liquid electrolyte containing Cl .
.
Mei, H.; Aoyagi, Noboru; Saito, Takumi*; Tanaka, Kazuya; Sugiura, Yuki; Tachi, Yukio
Applied Geochemistry, 162, p.105926_1 - 105926_8, 2024/02
Times Cited Count:2 Percentile:64.73(Geochemistry & Geophysics) Pt-labeled platinum complex and evaluation of its biodistribution in healthy mice
Pt-labeled platinum complex and evaluation of its biodistribution in healthy miceOmokawa, Marina*; Kimura, Hiroyuki*; Hatsukawa, Yuichi*; Kawashima, Hidekazu*; Tsukada, Kazuaki; Yagi, Yusuke*; Naito, Yuki*; Yasui, Hiroyuki*
Bioorganic & Medicinal Chemistry, 97, p.117557_1 - 117557_6, 2024/01
Times Cited Count:0 Percentile:0.00(Biochemistry & Molecular Biology)Massey, D.*; Williams, C. D.*; Mu, J.*; Masters, A. J.*; Motokawa, Ryuhei; Aoyagi, Noboru; Ueda, Yuki; Antonio, M. R.*
Journal of Physical Chemistry B, 127(9), p.2052 - 2065, 2023/03
Times Cited Count:2 Percentile:14.56(Chemistry, Physical)Hirata, Sakiko*; Kusaka, Ryoji; Meiji, Shogo*; Tamekuni, Seita*; Okudera, Kosuke*; Hamada, Shoken*; Sakamoto, Chihiro*; Honda, Takumi*; Matsushita, Kosuke*; Muramatsu, Satoru*; et al.
Inorganic Chemistry, 62(1), p.474 - 486, 2023/01
Times Cited Count:3 Percentile:27.89(Chemistry, Inorganic & Nuclear)Kusaka, Ryoji; Watanabe, Masayuki
Journal of Physical Chemistry Letters (Internet), 13(30), p.7065 - 7071, 2022/08
Times Cited Count:10 Percentile:71.30(Chemistry, Physical) O
O -pentadentate planar ligands designed for the strongest and selective capture of uranium from seawater
-pentadentate planar ligands designed for the strongest and selective capture of uranium from seawaterMizumachi, Takumi*; Sato, Minami*; Kaneko, Masashi; Takeyama, Tomoyuki*; Tsushima, Satoru*; Takao, Koichiro*
Inorganic Chemistry, 61(16), p.6175 - 6181, 2022/04
Times Cited Count:6 Percentile:52.57(Chemistry, Inorganic & Nuclear)Based on unique 5-fold equatorial coordination of UO
 , water-compatible pentadentate planar ligands, H
, water-compatible pentadentate planar ligands, H saldian and its derivatives, were designed as strong and selective capture of UO
saldian and its derivatives, were designed as strong and selective capture of UO
 in seawater. In the simulated seawater condition (0.5 M NaCl + 2.3 mM HCO
in seawater. In the simulated seawater condition (0.5 M NaCl + 2.3 mM HCO
 /CO
/CO
 , pH 8), saldian
, pH 8), saldian shows the strongest complexation with UO
shows the strongest complexation with UO
 to form UO
to form UO (saldian) (log
(saldian) (log
 = 28.05
= 28.05  0.07), which is more than 10 order of magnitude greater than amidoxime-based or -inspired ligand systems most commonly employed for U capture from seawater. Good selectivity for UO
0.07), which is more than 10 order of magnitude greater than amidoxime-based or -inspired ligand systems most commonly employed for U capture from seawater. Good selectivity for UO
 from other metal ions coexisting in seawater was also demonstrated.
from other metal ions coexisting in seawater was also demonstrated.
G tz, M.*; Yakushev, A.*; G
tz, M.*; Yakushev, A.*; G tz, S.*; Di Nitto, A.*; D
tz, S.*; Di Nitto, A.*; D llmann, Ch. E.*; Asai, Masato; Kindler, B.*; Krier, J.*; Lommel, B.*; Nagame, Yuichiro*; et al.
llmann, Ch. E.*; Asai, Masato; Kindler, B.*; Krier, J.*; Lommel, B.*; Nagame, Yuichiro*; et al.
Radiochimica Acta, 110(2), p.75 - 86, 2022/02
Times Cited Count:2 Percentile:20.20(Chemistry, Inorganic & Nuclear)The study of volatile superheavy element carbonyl complexes requires more efficient methods because the yield of transactinide elements decreases with increasing atomic number. This is achieved by using a newly developed double chamber system to separate the recoil chamber and the reaction one, thereby avoiding the decomposition of reactive molecules by the projectile ion beam, which hinders the synthesis of carbonyl complexes. The feasibility of this method was verified by synthesizing 5d metal short-lived isotopes as homologous element isotopes of the light transactinide elements Sg, Bh, Hs, and Mt at the Japan Atomic Energy Agency tandem accelerator and conducting model experiments.
Kusaka, Ryoji; Watanabe, Masayuki
Journal of Physical Chemistry B, 125(24), p.6727 - 6731, 2021/06
Times Cited Count:13 Percentile:46.94(Chemistry, Physical)Kwon, H.*; Pietrasiak, E.*; Ohara, Takashi; Nakao, Akiko*; Chae, B.*; Hwang, C.-C.*; Jung, D.*; Hwang, I.-C.*; Ko, Y. H.*; Kim, K.*; et al.
Inorganic Chemistry, 60(9), p.6403 - 6409, 2021/05
Times Cited Count:0 Percentile:0.00(Chemistry, Inorganic & Nuclear)G tz, M.*; G
tz, M.*; G tz, S.*; Kratz, J.-V.*; Ballof, J.*; D
tz, S.*; Kratz, J.-V.*; Ballof, J.*; D llmann, Ch. E.*; Eberhardt, K.*; Mokry, C.*; Renisch, D.*; Runke, J.*; Sato, Tetsuya; et al.
llmann, Ch. E.*; Eberhardt, K.*; Mokry, C.*; Renisch, D.*; Runke, J.*; Sato, Tetsuya; et al.
Radiochimica Acta, 109(3), p.153 - 165, 2021/03
Times Cited Count:3 Percentile:30.71(Chemistry, Inorganic & Nuclear)We report on a novel two-chamber approach for the synthesis of volatile complexes that allows spatial decoupling of thermalization and gas-phase carbonyl complex synthesis. Neutron induced fission on  U and spontaneous fission of
U and spontaneous fission of  Cm were employed for the production of the fission products. These were stopped inside a gas volume behind the target and flushed with an inert-gas flow into a second chamber. This was flushed with carbon monoxide to allow the gas-phase synthesis of carbonyl complexes. Parameter studies of the transfer from the first into the second chamber as well as on the carbonyl complex formation and transport processes have been performed. High overall efficiencies of more than 50% were reached rendering this approach interesting for studies of superheavy elements. Our results show that carbonyl complex formation of thermalized fission products is a single-atom reaction, and not a hot-atom reaction.
Cm were employed for the production of the fission products. These were stopped inside a gas volume behind the target and flushed with an inert-gas flow into a second chamber. This was flushed with carbon monoxide to allow the gas-phase synthesis of carbonyl complexes. Parameter studies of the transfer from the first into the second chamber as well as on the carbonyl complex formation and transport processes have been performed. High overall efficiencies of more than 50% were reached rendering this approach interesting for studies of superheavy elements. Our results show that carbonyl complex formation of thermalized fission products is a single-atom reaction, and not a hot-atom reaction.
 O
O -type Schiff base complexes of tetravalent f-elements (M(IV) = Ce, Th, U, Np, and Pu) in solid state and in solution
-type Schiff base complexes of tetravalent f-elements (M(IV) = Ce, Th, U, Np, and Pu) in solid state and in solutionRadoske, T.*; Kloditz, R.*; Fichter, S.*; M rz, J.*; Kaden, P.*; Patzschke, M.*; Schmidt, M.*; Stumpf, T.*; Walter, O.*; Ikeda, Atsushi
rz, J.*; Kaden, P.*; Patzschke, M.*; Schmidt, M.*; Stumpf, T.*; Walter, O.*; Ikeda, Atsushi
Dalton Transactions (Internet), 49(48), p.17559 - 17570, 2020/12
Times Cited Count:17 Percentile:74.45(Chemistry, Inorganic & Nuclear)Suno, Hiroya; Machida, Masahiko
RIST News, (66), p.3 - 16, 2020/10
no abstracts in English
Kusaka, Ryoji
Journal of Nuclear and Radiochemical Sciences (Internet), 20, p.28 - 31, 2020/06
Motokawa, Ryuhei; Kobayashi, Toru; Endo, Hitoshi; Mu, J.*; Williams, C. D.*; Masters, A. J.*; Antonio, M. R.*; Heller, W. T.*; Nagao, Michihiro*
ACS Central Science, 5(1), p.85 - 96, 2019/01
Times Cited Count:56 Percentile:85.91(Chemistry, Multidisciplinary) glycoprotein; Microbial transformation of heavy elements in the aquatic environment
glycoprotein; Microbial transformation of heavy elements in the aquatic environmentKozai, Naofumi; Sakamoto, Fuminori; Tanaka, Kazuya; Onuki, Toshihiko; Sato, Takahiro*; Kamiya, Tomihiro*; Grambow, B.
Chemosphere, 196, p.135 - 144, 2018/04
Times Cited Count:6 Percentile:17.62(Environmental Sciences)Transformation of heavy elements by microbes such as bacteria and fungi has been an intense research subject; however, little is known about that of protozoa. This study investigated interaction of a representative protozoa,  , with heavy elements (Eu(III), Pb(II), U(VI)). Non-destructive elemental analysis by micro-PIXE hardly detected those elements on living cells after sorption experiments but clearly detected on the cells that were killed with a fixative beforehand. Chromatographic analysis of aquatic species of those heavy elements after the sorption experiments revealed a fraction of those elements bound to a glycoprotein dissolved from the cell surface of living
, with heavy elements (Eu(III), Pb(II), U(VI)). Non-destructive elemental analysis by micro-PIXE hardly detected those elements on living cells after sorption experiments but clearly detected on the cells that were killed with a fixative beforehand. Chromatographic analysis of aquatic species of those heavy elements after the sorption experiments revealed a fraction of those elements bound to a glycoprotein dissolved from the cell surface of living  cells to form soluble pseudocolloid. These findings suggest that complexation of heavy elements with the dissolved surface glycoprotein reduced the sorption of those heavy elements on living cells.
cells to form soluble pseudocolloid. These findings suggest that complexation of heavy elements with the dissolved surface glycoprotein reduced the sorption of those heavy elements on living cells.
Motokura, Ken*; Fukuda, Takuma*; Uemura, Yohei*; Matsumura, Daiju; Ikeda, Marika*; Nambo, Masayuki*; Chun, W.-J.*
Catalysts, 8(3), p.106_1 - 106_8, 2018/03
Times Cited Count:4 Percentile:8.25(Chemistry, Physical) ssbauer isomer shifts with density functional calculations
ssbauer isomer shifts with density functional calculationsKaneko, Masashi; Watanabe, Masayuki; Miyashita, Sunao*; Nakashima, Satoru*
Radioisotopes, 66(8), p.289 - 300, 2017/08
Scalar-relativistic density functional calculations applied to some trivalent europium complexes. Five Eu(III) complexes whose 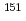 Eu M
Eu M ssbauer isomer shifts vary from -1.8 to 0.5 mm/s are referred by previously reported results. Geometrical optimizations of their complexes reproduces the experimental coordination structures. Single-point calculations are applied to their optimized geometries at three density functionals, namely, BP86, B3LYP, and B2PLYP, to obtain their electron densities at Eu nucleus. A comparison of the linearity between the electron densities and the corresponding
ssbauer isomer shifts vary from -1.8 to 0.5 mm/s are referred by previously reported results. Geometrical optimizations of their complexes reproduces the experimental coordination structures. Single-point calculations are applied to their optimized geometries at three density functionals, namely, BP86, B3LYP, and B2PLYP, to obtain their electron densities at Eu nucleus. A comparison of the linearity between the electron densities and the corresponding 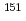 Eu M
Eu M ssbauer isomer shifts reveals that B2PLYP functional shows the best linearity. Electron population and bond analyses indicate that d- and f-orbital electrons of Eu ion in the complexes are found to be correlated to the experimental
ssbauer isomer shifts reveals that B2PLYP functional shows the best linearity. Electron population and bond analyses indicate that d- and f-orbital electrons of Eu ion in the complexes are found to be correlated to the experimental 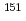 Eu M
Eu M ssbauer isomer shifts. This indicates that the d- and f-orbital electrons are involved in the covalent interaction of the coordination bond between the Eu ion and the ligands.
ssbauer isomer shifts. This indicates that the d- and f-orbital electrons are involved in the covalent interaction of the coordination bond between the Eu ion and the ligands.
 ssbauer spectroscopic parameters
ssbauer spectroscopic parametersKaneko, Masashi
Hosha Kagaku, (35), p.36 - 39, 2017/03
This paper is an article for research introduction by winner of the Japan Society of Nuclear and Radiochemical Sciences Encouragement Price 2016. It was mentioned about the achievements which revealed the spin transition behavior of iron complex and the separation mechanism of actinides from lanthanides.
Nakashima, Satoru*; Kaneko, Masashi
Advances in Chemistry Research, Vol.36, p.171 - 195, 2017/01
Spin-crossover (SCO) phenomena of the assembled coordination polymers are introduced. When the bridging ligand is flexible like 1,2-bis(4-pyridyl)ethane, 1,3-bis(4-pyridyl)propane, a variety of assembled structure can be obtained, depending on the conformer of the ligand and the guest molecules. Guest-dependent SCO phenomena of the assembled iron complexes are shown. Density functional theory is applied to know the cause of guest-dependent SCO phenomena. The validity of an iron mono-nucleus model is evaluated for the coordination polymers. It is shown that SCO occurs or not depends on the local structure around iron ion.