Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Soma, Yasutaka; Komatsu, Atsushi; Kaji, Yoshiyuki; Yamamoto, Masahiro*; Igarashi, Takahiro
Corrosion Science, 251, p.112897_1 - 112897_15, 2025/07
Times Cited Count:1 Percentile:0.00(Materials Science, Multidisciplinary)Experimental and modeling studies of the oxygen ingression at the crevices of stainless steels were conducted in high-temperature water (288 C). The limiting distance of oxygen ingression,
C). The limiting distance of oxygen ingression, 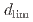 , was defined as the point beyond which the primary surface oxide changed (hematite
, was defined as the point beyond which the primary surface oxide changed (hematite  magnetite), regardless of crevice gap, oxygen concentration, and time. In situ measurements revealed increased electrical conductivity around the
magnetite), regardless of crevice gap, oxygen concentration, and time. In situ measurements revealed increased electrical conductivity around the 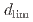 position indicating ion enrichment due to a differential oxygen concentration cell.
position indicating ion enrichment due to a differential oxygen concentration cell. 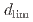 increased with increasing crevice gap, oxygen concentration, and immersion time. Modeling study suggested that oxide layer growth reduced anodic dissolution and slowed oxygen consumption, allowing oxygen ingression with time.
increased with increasing crevice gap, oxygen concentration, and immersion time. Modeling study suggested that oxide layer growth reduced anodic dissolution and slowed oxygen consumption, allowing oxygen ingression with time.
Soma, Yasutaka; Komatsu, Atsushi; Ueno, Fumiyoshi
Corrosion, 78(6), p.503 - 515, 2022/06
Times Cited Count:1 Percentile:7.22(Materials Science, Multidisciplinary)The effects of electrochemical potential (ECP) on water chemistry within a crevice are of critical importance for understanding stress corrosion cracking (SCC) of Fe-Cr-Ni alloys in high temperature water. In this study, the effects of ECP on the electrical conductivity of a solution within a Type-316L stainless steel crevice (
 ) have been studied in 288
) have been studied in 288 C and 8 MPa water containing 10 ppb Cl
C and 8 MPa water containing 10 ppb Cl as major anionic species. In situ measurements of
as major anionic species. In situ measurements of 
 in a rectangular crevice with a gap of 15
in a rectangular crevice with a gap of 15  m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in
m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in 
 from 12
from 12  Scm
Scm to 160
to 160  Scm
Scm at a distance of 21 mm from the crevice mouth. The increase in
at a distance of 21 mm from the crevice mouth. The increase in 
 reached a maximum at about 0.15 V (about 300
reached a maximum at about 0.15 V (about 300  Scm
Scm ) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl
) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl is the major anionic species transported into the crevice at relatively low potentials, and that
is the major anionic species transported into the crevice at relatively low potentials, and that 
 increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
 generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
Soma, Yasutaka; Kato, Chiaki
Zairyo To Kankyo 2022 Koenshu (CD-ROM), p.219 - 220, 2022/05
It is important to understand the electrochemical properties of stainless steel in environment created within crevice of stainless steel in high temperature water (crevice environment). This is because acidification and concentration of impurity ions occur in the crevice environment and this is common inside the stress corrosion crack. In this study, we reproduced the crevice environment in bulk scale and investigated mainly the effect of Cr concentration on the electrochemical properties of Fe-Cr-Ni alloys. Polarization curves of Fe-20Ni-xCr (x=16.4, 23, 26) were measured in water with a temperature of 288 C, a Cl concentration of 2
C, a Cl concentration of 2 10
10 mol/dm
mol/dm , a pH value of about 4.5, and a dissolved hydrogen concentration of 10 ppb. The peak currents of active dissolution (at -400 mV) and passive current density (at -50 mV) for specimens with Cr concentrations x = 16.4, 23, and 26% were approximately 13.8, 15.9, 10.0
, a pH value of about 4.5, and a dissolved hydrogen concentration of 10 ppb. The peak currents of active dissolution (at -400 mV) and passive current density (at -50 mV) for specimens with Cr concentrations x = 16.4, 23, and 26% were approximately 13.8, 15.9, 10.0  Acm
Acm , and 18.4, 8.5, 8.5
, and 18.4, 8.5, 8.5  Acm
Acm , respectively. Although the current values of x=26 were slightly lower in both cases, it was concluded that there was no clear dependence of the polarization curve on Cr concentration in this environment.
, respectively. Although the current values of x=26 were slightly lower in both cases, it was concluded that there was no clear dependence of the polarization curve on Cr concentration in this environment.
Soma, Yasutaka; Komatsu, Atsushi; Ueno, Fumiyoshi
Zairyo To Kankyo, 67(9), p.381 - 385, 2018/09
In-situ measurement of electrical conductivity of solution within crevice of SUS316L stainless steel in 288 C water has been conducted with newly developed electrochemical sensor system. The sensor measures local electrical conductivity of crevice solution beneath the electrode (
C water has been conducted with newly developed electrochemical sensor system. The sensor measures local electrical conductivity of crevice solution beneath the electrode (
 ) with electrochemical impedance method. The sensors were installed at different positions within tapered crevice of SUS316L stainless steel. The crevice specimen with the sensors were immerged into 288
) with electrochemical impedance method. The sensors were installed at different positions within tapered crevice of SUS316L stainless steel. The crevice specimen with the sensors were immerged into 288 C, 8 MPa, pure oxygen saturated high purity water for 100 h.
C, 8 MPa, pure oxygen saturated high purity water for 100 h. 
 at a position with crevice gap of
at a position with crevice gap of  59.3
59.3 m was 8-11
m was 8-11 S/cm, least deviate from conductivity of 288
S/cm, least deviate from conductivity of 288 C pure water (4.4
C pure water (4.4 S/cm) and no localized corrosion occurred. On the contrary,
S/cm) and no localized corrosion occurred. On the contrary, 
 at a position with crevice gap of
at a position with crevice gap of  4.4
4.4 m increased with time and showed maximum value of
m increased with time and showed maximum value of  1600
1600 S/cm at 70 h. Localized corrosion occurred in the vicinity of this position. Thermodynamic equilibrium calculation showed
S/cm at 70 h. Localized corrosion occurred in the vicinity of this position. Thermodynamic equilibrium calculation showed 
 of 1600
of 1600 S/cm being equivalent to pH of 3 to 3.7. It can be concluded that acidification occurred in tight crevice even under high purity bulk water and resulted in localized corrosion.
S/cm being equivalent to pH of 3 to 3.7. It can be concluded that acidification occurred in tight crevice even under high purity bulk water and resulted in localized corrosion.
Soma, Yasutaka; Ueno, Fumiyoshi
Zairyo To Kankyo, 67(5), p.222 - 228, 2018/05
Localized corrosion in crevice of SUS316 stainless steel after immersion in 288 C high purity water with dissolved oxygen concentration of 32 ppm for 100 h was analyzed. Two different types of localized corrosion initiated on grain boundary and inclusions. The former initiated on grain boundary and oxide grown into grain matrix. The oxidized area showed duplex structure composed of microcrystalline FeCr
C high purity water with dissolved oxygen concentration of 32 ppm for 100 h was analyzed. Two different types of localized corrosion initiated on grain boundary and inclusions. The former initiated on grain boundary and oxide grown into grain matrix. The oxidized area showed duplex structure composed of microcrystalline FeCr O
O and island-shaped residual metals. The latter initiated on inclusions containing Ca and S and microcrystalline FeCr
and island-shaped residual metals. The latter initiated on inclusions containing Ca and S and microcrystalline FeCr O
O grown into metal matrix. These localized corrosion occurred selectively in oxygen depleted area indicated formation of macroscopic corrosion cell with the corroded area as anode and surrounding oxygenated area as cathode.
grown into metal matrix. These localized corrosion occurred selectively in oxygen depleted area indicated formation of macroscopic corrosion cell with the corroded area as anode and surrounding oxygenated area as cathode.
Takeuchi, Tomoaki; Nakano, Hiroko; Uehara, Toshiaki; Tsuchiya, Kunihiko
Nuclear Materials and Energy (Internet), 9, p.451 - 454, 2016/12
Times Cited Count:1 Percentile:9.49(Nuclear Science & Technology)no abstracts in English
Soma, Yasutaka; Kato, Chiaki; Ueno, Fumiyoshi
Fushoku Boshoku Kyokai Dai-63-Kai Zairyo To Kankyo Toronkai Koenshu (CD-ROM), p.253 - 256, 2016/10
Contribution of corrosion to advance of stress corrosion cracking (SCC) of stainless steel in high temperature water must be assessed because serious corrosion can be found within SCC of light water reactors. The corrosion took the form of both intergranular and grain-matrix attack indicate aggressive corrosion condition was formed in the crevice of the SCC. We have investigated the crevice environment electrochemically and found that local electrical conductivity of the crevice solution at satisfactory narrow crevice gap having more than 100 times higher than that of bulk solution. In this research we assessed effect of cyclic deaerated and aerated bulk solution to the crevice environment. The result showed that electrical conductivity of the crevice solution under the deaerated bulk solution increased more than 10times by injection of pure oxygen suggest that the dissolved oxygen caused aggressive corrosion condition within the crevice.
Nakano, Hiroko; Uehara, Toshiaki; Takeuchi, Tomoaki; Shibata, Hiroshi; Nakamura, Jinichi; Matsui, Yoshinori; Tsuchiya, Kunihiko
JAEA-Technology 2015-049, 61 Pages, 2016/03
In Japan Atomic Energy Agency, we started a research and development so as to monitor the Nuclear Plant Facilities situations during a severe accident, such as a radiation-resistant monitoring camera under a severe accident, a radiation resistant in-water transmission system for conveying the information in-core and a heat-resistant signal cable. As part of advance in a heat-resistant signal cable, we maintained to ex-core high-temperature and pressure water loop test equipment which can be simulated conditions of BWRs and PWRs for evaluation reliability and property of construction sheath materials. This equipment consists of Autoclave, water conditioning tank, water pump, high-pressure metering pump, preheater, heat exchanger and pure water purification equipment. This report describes the basic design and the results of performance tests of construction machinery and tools of ex-core high-temperature and pressure water loop test equipment.
Kimura, Takaumi; Kirishima, Akira*; Arisaka, Makoto
Kidorui, (47), p.43 - 56, 2005/11
no abstracts in English
Tsukada, Takashi; Miwa, Yukio; Ugachi, Hirokazu; Matsui, Yoshinori; Itabashi, Yukio; Nagata, Nobuaki*; Dozaki, Koji*
Proceedings of International Conference on Water Chemistry of Nuclear Reactor Systems (CD-ROM), 5 Pages, 2004/10
IASCC initiation and propagation tests will be performed on the per-irradiated specimen in the Japan Materials Testing Reactor (JMTR). Since in core, the radiolysis of water causes a generation of various kind of radical species and some oxidizing species such as hydrogen peroxide, the water chemistry in irradiation capsules must be assessed by measurements of the electrochemical corrosion potential (ECP). For the in-core measurement of ECP in JMTR, we fabricated and tested the Fe/Fe O
O type ECP sensor. After the fabrication, the function of each sensor was examined in high temperature water by out-of-core thermal cycling and high temperature holding tests.
type ECP sensor. After the fabrication, the function of each sensor was examined in high temperature water by out-of-core thermal cycling and high temperature holding tests.
Kimura, Takaumi; Nagaishi, Ryuji; Arisaka, Makoto*; Ozaki, Takuo; Yoshida, Zenko
Radiochimica Acta, 90(9-11), p.715 - 719, 2002/11
Times Cited Count:15 Percentile:66.70(Chemistry, Inorganic & Nuclear)no abstracts in English
Tsukada, Takashi
Nihon Yosetsu Kyokai "Genshiryoku Kozo Kiki No Zairyo, Sekkei, Seko, Kensa Ni Kansuru Koshukai" Tekisuto, 40 Pages, 2002/00
no abstracts in English
Tsukada, Takashi; Ebine, Noriya
Nihon AEM Gakkai-Shi, 9(2), p.171 - 177, 2001/06
no abstracts in English
Ouchi, Nobuo; Kusano, Joichi; Akaoka, Nobuo*; Mizumoto, Motoharu; Inoue, Hitoshi*; Noguchi, Shuichi*; Saito, Kenji*; Takeda, O.*; Murai, T.*; Kijima, Y.*; et al.
KEK Proceedings 99-25, p.33 - 37, 2000/02
no abstracts in English
Tsukada, Takashi
Proceedings of Seminar on Water Chemistry of Nuclear Reactor Systems '99, p.26 - 32, 1999/00
no abstracts in English
Tsukada, Takashi
JAERI-Research 98-007, 187 Pages, 1998/03
no abstracts in English
; Hasegawa, Kazuo; Honda, Yoichiro*; Kusano, Joichi; Mizumoto, Motoharu; Mukugi, Ken*; Ouchi, Nobuo; Inoue, Hitoshi*; Noguchi, Shuichi*; Saito, Kenji*
Proceedings of 6th European Particle Accelerator Conference (EPAC98) (CD-ROM), p.734 - 736, 1998/01
no abstracts in English
Matsui, Yoshinori; Niimi, Motoji; Hoshiya, Taiji; Tsukada, Takashi; Tsuji, Hirokazu
Journal of Nuclear Materials, 258-263, p.378 - 382, 1998/00
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)no abstracts in English
Akaoka, Nobuo*; Kusano, Joichi; Ouchi, Nobuo; Mizumoto, Motoharu; Takeda, O.*; Noguchi, Shuichi*; Saito, Kenji*; ; ;
Proceedings of 23rd Linear Accelerator Meeting in Japan, p.289 - 291, 1998/00
no abstracts in English
Kusano, Joichi; Ouchi, Nobuo; Akaoka, Nobuo*; ; Noguchi, Shuichi*; Mukugi, Ken*; Hasegawa, Kazuo; Mizumoto, Motoharu
Proc. of 11th Symp. on Accelerator Sci. and Technol., p.240 - 242, 1997/00
no abstracts in English