Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Minowa, Kazuki*; Watanabe, So; Nakase, Masahiko*; Takahatake, Yoko; Miyazaki, Yasunori; Ban, Yasutoshi; Matsuura, Haruaki*
Nuclear Instruments and Methods in Physics Research B, 556, p.165496_1 - 165496_6, 2024/11
Times Cited Count:0 Percentile:0.00(Instruments & Instrumentation)In this study, X-ray absorption near edge structure (XANES) spectral analysis and column experiments were used to verify the selectivity of rare earth (RE) ions by alkyl diamide amine (ADAAM) adsorbent. In addition, the interactions between the N atoms of ADAAM and RE ions were evaluated to determine whether any of the RE ions are a valid simulant for developing a mutual separation process for minor actinides (MAs) in highly radioactive liquid waste. It was confirmed that La and Ce interacted with the amine N atom of ADAAM and they showed a peak shift of the N-K edge XANES spectrum; this finding suggested that a soft interaction is an essential factor influencing ion selectivity. Therefore, the selection factor of RE ions by ADAAM adsorbent was similar to that of MAs. It was concluded that RE ions are reasonable species to simulate MAs.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Hideya*
Journal of Nuclear Science and Technology, 61(7), p.883 - 893, 2024/07
Times Cited Count:4 Percentile:59.85(Nuclear Science & Technology)The mutual separation of Am and Cm is conducted using an alkyl-diamide amine (ADAAM) extractant. ADAAM exhibits extremely high separation factor with respect to Am and Cm separation (5.9) in a nitric acid- -dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
-dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
Kinoshita, Ryoma; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Hydrometallurgy, 222, p.106159_1 - 106159_12, 2023/10
Times Cited Count:2 Percentile:23.52(Metallurgy & Metallurgical Engineering)Solvent extraction is conducted using a total of 20 metals revealing high stability constants with Cl and hexahexyl-nitrilotriacetamide (NTAamide(C6)) extractant. The metals used here may behave as anions at high Cl concentrations, and NTAamide(C6), which contains a tertiary N atom, is protonated under acidic conditions. Most of the metal ions in this study display higher distribution ratios (D(M)) from HCl than those from HNO , and exhibit 1:1 stoichiometries with NTAamide. Following the experimental results, the association constants and distribution coefficients of the group 12 elements are calculated via ion-pair extraction modeling using density functional theory calculations, and the simulations of D yield calculated values with the same trend as that of the measured values.
, and exhibit 1:1 stoichiometries with NTAamide. Following the experimental results, the association constants and distribution coefficients of the group 12 elements are calculated via ion-pair extraction modeling using density functional theory calculations, and the simulations of D yield calculated values with the same trend as that of the measured values.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:3 Percentile:35.90(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)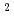
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
Suzuki, Hideya*; Ban, Yasutoshi
Analytical Sciences, 39(8), p.1341 - 1348, 2023/08
Times Cited Count:4 Percentile:46.80(Chemistry, Analytical)The Japan Atomic Energy Agency (JAEA) has proposed the Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation (SELECT) process by solvent extraction as a new separation technology to recover minor actinides (MA) from high-level liquid waste (HLLW) produced by spent fuel reprocessing. The MA separation in the SELECT process comprises the batch recovery of MA and rare earths (RE) from HLLW, MA/RE separation, and Am/Cm separation. Three highly practical extractants are used in the MA separation. Furthermore, this flow configuration facilitates the preparation of nitric acid concentrations in the aqueous phase. However, the separation factor between Cm and Nd in the MA/RE separation is small (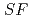
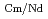 = 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
= 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
Kaneko, Masashi; Suzuki, Hideya; Matsumura, Tatsuro
Inorganic Chemistry, 57(23), p.14513 - 14523, 2018/12
Times Cited Count:24 Percentile:79.50(Chemistry, Inorganic & Nuclear)We elucidated the separation mechanism between Am(III) and Cm(III) ions by using two different types of diamide ligands, diglycolamide (DGA) and alkylated diamide amine (ADAAM), by means of the density functional theory technique and electron density analysis. The molecular geometries and formation reactions of the metal-ligand complexes were modeled by using [M(DGA) ]
] and [M(ADAAM)(NO
and [M(ADAAM)(NO )
) (H
(H O)]. We successfully reproduced Cm(III) selectivity over Am(III) with DGA and Am(III) selectivity over Cm(III) with ADAAM. Furthermore, we analyzed the bonding properties between the metal ion and the diamide-type ligands by using model complexes, [M(DGA)
O)]. We successfully reproduced Cm(III) selectivity over Am(III) with DGA and Am(III) selectivity over Cm(III) with ADAAM. Furthermore, we analyzed the bonding properties between the metal ion and the diamide-type ligands by using model complexes, [M(DGA) ]
] and [M(ADAAM)(NO
and [M(ADAAM)(NO )
) (H
(H O)], and revealed the differences in terms of the bond dissociation energy and the metal 5f-orbital participation in the covalency between the Am(III) and the Cm(III) complexes. It was suggested that the differences were key factors to understand the Am(III)/Cm(III) selectivity.
O)], and revealed the differences in terms of the bond dissociation energy and the metal 5f-orbital participation in the covalency between the Am(III) and the Cm(III) complexes. It was suggested that the differences were key factors to understand the Am(III)/Cm(III) selectivity.
Sasaki, Yuji; Morita, Keisuke; Ito, Keisuke; Suzuki, Shinichi; Shiwaku, Hideaki; Takahashi, Yuya*; Kaneko, Masaaki*; Omori, Takashi*; Asano, Kazuhito*
Proceedings of International Nuclear Fuel Cycle Conference (GLOBAL 2017) (USB Flash Drive), 4 Pages, 2017/09
no abstracts in English
Ogura, K.*; Asano, Masaharu; Yasuda, Nakahiro*; Yoshida, Masaru
Nuclear Instruments and Methods in Physics Research B, 185(1-4), p.222 - 227, 2001/12
Times Cited Count:33 Percentile:88.94(Instruments & Instrumentation)no abstracts in English
Hiraki, Yoshihisa; Terasawa, Toshiharu*; Imaizumi, Ken*; Taniguchi, Takumi; Kato, Jun; Osugi, Takeshi; Sone, Tomoyuki; Nakazawa, Osamu; Kuroki, Ryoichiro
no journal, ,
The relationship between the amount of radionuclide to be solidified and the solidified body temperature was analyzed, when solidify contaminated water management waste at Fukushima Daiichi Nuclear Power Station with cement etc. at low temperature. The analysis code used the radiation transport code and the thermal analysis code. Thus, the limit value by radionuclide concentration during solidification process are evaluated. The summary of the test and some of the obtained results are introduced.
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Toigawa, Tomohiro
no journal, ,
To minimize the radioactive waste from nuclear fuel cycle, we have conducted research and development of the new reprocessing and MA separation process, SELECT process (Solvent Extraction from Liquid-waste using Extractants of CHON-type for Transmutation), using innovative extractants. The extractants for each solvent extraction processes were developed in JAEA. The extractants for reprocessing process are monoamides as alternative extractants for TBP. For MA+RE recovery process, we developed TDdDGA which has very high performance to recover of MA from high level waste. HONTA and ADAAM were developed for MA/RE separation process and Am/Cm separation process respectively. All of the extractants consist of C, H, O, and N elements, and can be decomposed to gases by incineration. The demonstration tests using genuine spent fuel and high level liquid waste of the SELECT process have been conducted. Uranium solution and U+Pu mixed solution were separated from spent fuel, and MA, americium and curium, were recovered and separated from HLW effectively. The details of the extraction tests for each separation processes will be presented in correspond presentations in this conference.
Suzuki, Hideya*; Ban, Yasutoshi; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; Kawasaki, Tomohiro*; Matsumura, Tatsuro
no journal, ,
A highly practical hybrid-type (soft  -donor and hard
-donor and hard 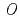 -donor) extractant, which is an alkyldiamideamine (ADAAM), was investigated for the minor actinides (MA) separation. The new process aims at recovering americium (Am) alone from high-level waste liquid (HLLW) using an ADAAM. The principle of the process is based on the extraction of Am together with light lanthanides (La, Ce, Pr and Nd) and Mo having close values of distribution ratio, while curium, other lanthanides, and other fission products remain in the aqueous phase. The Am was subsequently selectively stripped from the light lanthanides and Mo using mixed solution (DTPA, malonic acid and ammonium nitrate). As a result, Am was directly separated from the simulated HLLW with high yield (95%).
-donor) extractant, which is an alkyldiamideamine (ADAAM), was investigated for the minor actinides (MA) separation. The new process aims at recovering americium (Am) alone from high-level waste liquid (HLLW) using an ADAAM. The principle of the process is based on the extraction of Am together with light lanthanides (La, Ce, Pr and Nd) and Mo having close values of distribution ratio, while curium, other lanthanides, and other fission products remain in the aqueous phase. The Am was subsequently selectively stripped from the light lanthanides and Mo using mixed solution (DTPA, malonic acid and ammonium nitrate). As a result, Am was directly separated from the simulated HLLW with high yield (95%).
Kikuchi, Michio*; Yamamoto, Takeshi*; Otsuka, Taku*; Kawato, Takaya*; Kaneda, Yoshihisa*; Shibata, Masahito*; Haga, Kazuko*; Osugi, Takeshi; Sone, Tomoyuki; Kuroki, Ryoichiro
no journal, ,
In order to obtain data to be used in the evaluation of the applicability of low temperature processing to carbonate slurry generated by contaminated water treatment at Fukushima Daiichi Nuclear Power Station, the basic properties of the solidified cement and alkali activated material blended simulated carbonated slurry were evaluated. An overview of the study and some of the results obtained are reported here.
Kaneda, Yoshihisa*; Haga, Kazuko*; Shibata, Masahito*; Osawa, Norihisa*; Kikuchi, Michio*; Yamamoto, Takeshi*; Kawato, Takaya*; Osugi, Takeshi; Sone, Tomoyuki; Kuroki, Ryoichiro
no journal, ,
Solidified cement and alkali activated material blending carbonated slurry were prepared and their dissolution tests were carried out in order to obtain basic data for the low temperature processing of the waste generated by contaminated water treatment at Fukushima Daiichi Nuclear Power Station. An overview of the study and some of the results obtained are reported here.
Hiraki, Yoshihisa; Saito, Toshimitsu*; Kakuda, Ayaka; Osugi, Takeshi; Sone, Tomoyuki; Kuroki, Ryoichiro; Kudo, Isamu*; Elakneswaran, Y.*; Sato, Tsutomu*
no journal, ,
no abstracts in English
Okada, Makoto*; Watanabe, So; Nakase, Masahiko*; Ban, Yasutoshi; Shiwaku, Hideaki; Matsuura, Haruaki*
no journal, ,
no abstracts in English
Hiraki, Yoshihisa
no journal, ,
no abstracts in English
Suzuki, Hideya*; Shimojo, Kojiro; Nakamura, Satoshi; Emori, Tatsuya; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; Kawasaki, Tomohiro*; Ban, Yasutoshi
no journal, ,
The Japan Atomic Energy Agency (JAEA) has been studying partitioning and transmutation (P&T) systems. In P&T, JAEA proposed a novel hydrometallurgical process called "SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process" for nuclear fuel reprocessing and minor actinides (MA) separation. In this study, a new MA separation method was investigated to further improve the efficiency of the SELECT process. Batch tests were conducted in a mixed solvent system using the acidic amide-type extractants nitrilotriacetic acid diacetamide with branched side chains (B-TONAADA) and alkyl diamidoamine (ADAAM). As a result, we found an efficient method for separating only americium (Am) from an aqueous nitric acid solution containing MA and rare earth elements by adjusting nitric acid concentration.
Sakamoto, Ryo*; Kaneda, Yoshihisa*; Kobayashi, Yutaro*; Haga, Kazuko*; Taniguchi, Takumi; Kuroki, Ryoichiro; Osugi, Takeshi
no journal, ,
no abstracts in English
Kobayashi, Yutaro*; Osawa, Norihisa*; Haga, Kazuko*; Kaneda, Yoshihisa*; Chaerun Raudhatul, I.*; Sato, Tsutomu*; Taniguchi, Takumi; Kuroki, Ryoichiro; Osugi, Takeshi
no journal, ,
no abstracts in English
Taniguchi, Takumi; Imaizumi, Ken*; Namiki, Masahiro*; Osugi, Takeshi; Kuroki, Ryoichiro; Kikuchi, Michio*; Yamamoto, Takeshi*; Kaneda, Yoshihisa*; Haga, Kazuko*
no journal, ,
It is important to understand fundamental solidification characteristics of contaminated water management waste at Fukushima Daiichi Nuclear Power Station. The Solidified bodies are fabricated with cementitious material and Alkali Activated Material, and are irradiated with Gamma-ray.