Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Minowa, Kazuki*; Watanabe, So; Nakase, Masahiko*; Takahatake, Yoko; Miyazaki, Yasunori; Ban, Yasutoshi; Matsuura, Haruaki*
Nuclear Instruments and Methods in Physics Research B, 556, p.165496_1 - 165496_6, 2024/11
Times Cited Count:0 Percentile:0.00(Instruments & Instrumentation)In this study, X-ray absorption near edge structure (XANES) spectral analysis and column experiments were used to verify the selectivity of rare earth (RE) ions by alkyl diamide amine (ADAAM) adsorbent. In addition, the interactions between the N atoms of ADAAM and RE ions were evaluated to determine whether any of the RE ions are a valid simulant for developing a mutual separation process for minor actinides (MAs) in highly radioactive liquid waste. It was confirmed that La and Ce interacted with the amine N atom of ADAAM and they showed a peak shift of the N-K edge XANES spectrum; this finding suggested that a soft interaction is an essential factor influencing ion selectivity. Therefore, the selection factor of RE ions by ADAAM adsorbent was similar to that of MAs. It was concluded that RE ions are reasonable species to simulate MAs.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Hideya*
Journal of Nuclear Science and Technology, 61(7), p.883 - 893, 2024/07
Times Cited Count:3 Percentile:62.75(Nuclear Science & Technology)The mutual separation of Am and Cm is conducted using an alkyl-diamide amine (ADAAM) extractant. ADAAM exhibits extremely high separation factor with respect to Am and Cm separation (5.9) in a nitric acid- -dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
-dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
Suzuki, Hideya*; Ban, Yasutoshi
Analytical Sciences, 39(8), p.1341 - 1348, 2023/08
Times Cited Count:4 Percentile:49.52(Chemistry, Analytical)The Japan Atomic Energy Agency (JAEA) has proposed the Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation (SELECT) process by solvent extraction as a new separation technology to recover minor actinides (MA) from high-level liquid waste (HLLW) produced by spent fuel reprocessing. The MA separation in the SELECT process comprises the batch recovery of MA and rare earths (RE) from HLLW, MA/RE separation, and Am/Cm separation. Three highly practical extractants are used in the MA separation. Furthermore, this flow configuration facilitates the preparation of nitric acid concentrations in the aqueous phase. However, the separation factor between Cm and Nd in the MA/RE separation is small (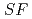
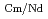 = 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
= 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
Kaneko, Masashi; Suzuki, Hideya; Matsumura, Tatsuro
Inorganic Chemistry, 57(23), p.14513 - 14523, 2018/12
Times Cited Count:24 Percentile:79.73(Chemistry, Inorganic & Nuclear)We elucidated the separation mechanism between Am(III) and Cm(III) ions by using two different types of diamide ligands, diglycolamide (DGA) and alkylated diamide amine (ADAAM), by means of the density functional theory technique and electron density analysis. The molecular geometries and formation reactions of the metal-ligand complexes were modeled by using [M(DGA) ]
] and [M(ADAAM)(NO
and [M(ADAAM)(NO )
) (H
(H O)]. We successfully reproduced Cm(III) selectivity over Am(III) with DGA and Am(III) selectivity over Cm(III) with ADAAM. Furthermore, we analyzed the bonding properties between the metal ion and the diamide-type ligands by using model complexes, [M(DGA)
O)]. We successfully reproduced Cm(III) selectivity over Am(III) with DGA and Am(III) selectivity over Cm(III) with ADAAM. Furthermore, we analyzed the bonding properties between the metal ion and the diamide-type ligands by using model complexes, [M(DGA) ]
] and [M(ADAAM)(NO
and [M(ADAAM)(NO )
) (H
(H O)], and revealed the differences in terms of the bond dissociation energy and the metal 5f-orbital participation in the covalency between the Am(III) and the Cm(III) complexes. It was suggested that the differences were key factors to understand the Am(III)/Cm(III) selectivity.
O)], and revealed the differences in terms of the bond dissociation energy and the metal 5f-orbital participation in the covalency between the Am(III) and the Cm(III) complexes. It was suggested that the differences were key factors to understand the Am(III)/Cm(III) selectivity.
Kaneko, Masashi; Watanabe, Masayuki; Suzuki, Hideya; Matsumura, Tatsuro
no journal, ,
Japan Atomic Energy Agency has developed separation reagents between Am and Cm ions, which are minor actinides, to achieve partitioning and transmutation strategy. We aim to elucidate the Am/Cm separation mechanism by various reagents at a molecular level to design a novel extraction reagent. This study focused on the Am/Cm separation with diglycolamide (DGA) and alkyl-diacetoamidamine (ADAAM) reagents by using density functional theory. We modeled the complex formation reactions by referring to reported single crystal structures and solvent extraction experiments. As the result, we succeeded in reproducing the Am/Cm separation behaviors with DGA and ADAAM reagents. Moreover, the electronic state analyses of the extracted complexes indicated that a difference in chemical bonding property of 5f-electrons between Am and Cm differentiates the Am/Cm selectivity.
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Toigawa, Tomohiro
no journal, ,
To minimize the radioactive waste from nuclear fuel cycle, we have conducted research and development of the new reprocessing and MA separation process, SELECT process (Solvent Extraction from Liquid-waste using Extractants of CHON-type for Transmutation), using innovative extractants. The extractants for each solvent extraction processes were developed in JAEA. The extractants for reprocessing process are monoamides as alternative extractants for TBP. For MA+RE recovery process, we developed TDdDGA which has very high performance to recover of MA from high level waste. HONTA and ADAAM were developed for MA/RE separation process and Am/Cm separation process respectively. All of the extractants consist of C, H, O, and N elements, and can be decomposed to gases by incineration. The demonstration tests using genuine spent fuel and high level liquid waste of the SELECT process have been conducted. Uranium solution and U+Pu mixed solution were separated from spent fuel, and MA, americium and curium, were recovered and separated from HLW effectively. The details of the extraction tests for each separation processes will be presented in correspond presentations in this conference.
Suzuki, Hideya*; Ban, Yasutoshi; Tsubata, Yasuhiro; Tsutsui, Nao; Toigawa, Tomohiro; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; Kawasaki, Tomohiro*; Matsumura, Tatsuro
no journal, ,
The Japan Atomic Energy Agency (JAEA) has been studying partitioning technology. Recently, JAEA proposed a new liquid-liquid extraction technology called SELECT process to separate minor actinide (MA) from high-level liquid waste for transmutation. In this process, new extractants (HONTA, ADAAM) with highly practical and high extraction ability for MA was developed. A mixed solvent of HONTA and ADAAM was tested for mutual separation of MA and rare earth elements (RE). In this test, separation of MA and RE was achieved with very high yield. Furthermore, Americium (Am) and Curium (Cm) were separated efficiently with high separation factor values.
Suzuki, Hideya*; Ban, Yasutoshi; Tsubata, Yasuhiro; Hotoku, Shinobu; Toigawa, Tomohiro; Tsutsui, Nao; Shibata, Mitsunobu*; Kurosawa, Tatsuya*; Kawasaki, Tomohiro*; Matsumura, Tatsuro
no journal, ,
The Japan Atomic Energy Agency has been studying partitioning and transmutation (P&T) systems. In the P&T, the separation of minor actinides (MAs) from the chemically similar lanthanides is the key step. After MAs are separated from high-level waste, the mutual separation of Am and Cm (Am/Cm separation) can be conducted. Therefore, the removal of the pyrogenic Cm nuclide would reduce the difficulties associated with MA-fuel fabrication. However, Am/Cm separation is very challenging because the two elements have similar chemical and physical properties. Highly practical a new reagent, called ADAAM have been developed. The Am is subsequently selectively stripped from the light lanthanides. As a result, Am was separated with high efficiency.
Suzuki, Hideya*; Ban, Yasutoshi; Tsubata, Yasuhiro; Hotoku, Shinobu; Morita, Keisuke; Toigawa, Tomohiro; Tsutsui, Nao; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; Kawasaki, Tomohiro*; et al.
no journal, ,
The Japan Atomic Energy Agency has been studying partitioning and transmutation (P&T) systems. In the P&T, the separation of minor actinide (MA) from the chemically similar lanthanides is the key step. After MAs are separated from high-level liquid waste (HLLW), the mutual separation of Am and Cm (Am/Cm separation) can be conducted. Therefore, the removal of the pyrogenic Cm nuclide would reduce the difficulties associated with MA-fuel fabrication. However, Am/Cm separation is very challenging because the two elements have similar chemical and physical properties. Highly practical new reagents, called HONTA and ADAAM have been developed. Solvent extraction tests were performed using a mixture of HONTA and ADAAM. As a result, the separation of Am from the simulated HLLW was achieved with high yield.
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Toigawa, Tomohiro; Tsutsui, Nao; Hotoku, Shinobu; Suzuki, Asuka
no journal, ,
no abstracts in English
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Hideya*
no journal, ,
Am and Cm as minor actinides, have the similar chemical behavior, thus both metals are, in general, hard to separate. On the other hand, separation of Am with long-lived radionuclide to transmute from Cm to emit neutrons by spontaneous fission has been requesting. Although the mutual separation by solvent extraction is a tough work, ADAAM (Alkyl DiAmide AMine) developed in JAEA show high separation factor of Am/Cm of around 6 at HNO -n-dodecane system. We focus on basic research on solvent extraction using ADAAM and investigate the physical properties, i.e., polarity and viscosity, and separation factors of Am/Cm variated in combination with water-soluble masking agent of An, and will show the information at the meeting.
-n-dodecane system. We focus on basic research on solvent extraction using ADAAM and investigate the physical properties, i.e., polarity and viscosity, and separation factors of Am/Cm variated in combination with water-soluble masking agent of An, and will show the information at the meeting.
Matsumura, Tatsuro; Ban, Yasutoshi; Hotoku, Shinobu; Suzuki, Hideya; Tsubata, Yasuhiro; Tsutsui, Nao; Suzuki, Asuka
no journal, ,
Suzuki, Hideya*; Ban, Yasutoshi; Hotoku, Shinobu; Morita, Keisuke; Tsutsui, Nao; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; Kawasaki, Tomohiro*; Matsumura, Tatsuro
no journal, ,
The Japan Atomic Energy Agency (JAEA) has been studying partitioning technology. Recently, JAEA proposed a new liquid-liquid extraction technology called SELECT (Solvent Extraction from Liquid-waste using Extractants of CHON-type for Transmutation) process to separate minor actinide (MA) from high-level liquid waste (HLLW) for transmutation. In this process, new extractants (HONTA, ADAAM) with highly practical and high extraction ability for MA was developed. In the present study, a new solvent extraction method using a mixture of HONTA and ADAAM was investigated. In the tests, it was found that the separation of Am from the simulated HLLW was achieved with very high yield.
Sasaki, Yuji; Kaneko, Masashi; Suzuki, Hideya*; Ban, Yasutoshi
no journal, ,
ADAAM, which is a tridentate diamide including tertiary N atom and developed by JAEA, shows very high separation factor (SF=6) of Am/Cm in nitric acid-n-dodecane system. We tried to enlarge SF using masking agents, however, it is not successful up to now. Thus, the batchwise multi-stage extraction using solo ADAAM for Am/Cm separation is performed. One of the advantages for this technique is available to use small volumes of organic and aqueous phases. Calculation gives product of more than 95% Am with less than 5% Cm by 12 stages of solvent extraction. On the other hand, because of relatively low SF, volume of the raffinate solution may increase several times after multi-stage extraction. In this presentation, we discuss how to cut down their volumes.
Minowa, Kazuki*; Watanabe, So; Ban, Yasutoshi; Nakase, Masahiko*; Watanabe, Shinta*; Matsuura, Haruaki*
no journal, ,
no abstracts in English
Sasaki, Yuji; Ban, Yasutoshi
no journal, ,
JAEA has developed DGA compounds for total recovery of Ln+An, MIDOA (methylimino-dioctylacetamide) and NTAamide (nitrilotriacetamide) for extractions of Mo, Tc and Pd, ADAAM (alkyldiamide amine) for Am/Cm separation, and those extractants are considered to be outstanding in the world. However, some of the prominent reagents are examined in the same separation purpose, so it is difficult to select the suitable reagents. In this work, we evaluated the utilities of extractants and masking agents taking into account the linkage among three processes and the concentrations of metal ions in high-level radioactive waste. Thus, conditions of extraction process for total recovery of Ln+An using several DGA compounds and DOODA (dioxaoctanediamide), Ln/An separation process using NTAamide and DTBA, and Am/Cm separation process using ADAAM and DGA/DOODA are compared. Following the results in this work, the simple and effective separation scheme is investigated.
Suzuki, Hideya*; Ban, Yasutoshi; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; Kawasaki, Tomohiro*; Matsumura, Tatsuro
no journal, ,
A highly practical hybrid-type (soft  -donor and hard
-donor and hard  -donor) extractant, which is an alkyldiamideamine (ADAAM), was investigated for the minor actinides (MA) separation. The new process aims at recovering americium (Am) alone from high-level waste liquid (HLLW) using an ADAAM. The principle of the process is based on the extraction of Am together with light lanthanides (La, Ce, Pr and Nd) and Mo having close values of distribution ratio, while curium, other lanthanides, and other fission products remain in the aqueous phase. The Am was subsequently selectively stripped from the light lanthanides and Mo using mixed solution (DTPA, malonic acid and ammonium nitrate). As a result, Am was directly separated from the simulated HLLW with high yield (95%).
-donor) extractant, which is an alkyldiamideamine (ADAAM), was investigated for the minor actinides (MA) separation. The new process aims at recovering americium (Am) alone from high-level waste liquid (HLLW) using an ADAAM. The principle of the process is based on the extraction of Am together with light lanthanides (La, Ce, Pr and Nd) and Mo having close values of distribution ratio, while curium, other lanthanides, and other fission products remain in the aqueous phase. The Am was subsequently selectively stripped from the light lanthanides and Mo using mixed solution (DTPA, malonic acid and ammonium nitrate). As a result, Am was directly separated from the simulated HLLW with high yield (95%).
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Toigawa, Tomohiro; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; et al.
no journal, ,
To continue the utilization of the nuclear fission energy, the management of the high-level radioactive waste is one of the most important issues to be solved. Partitioning and Transmutation technology is expected to be effective to mitigate the burden of the HLW disposal by reducing the radiological toxicity and heat generation. JAEA has been conducting R&D on the MA separation process to remove of MA from HLW and supply the recovered MA to the transmutation system such as ADS. The MA separation process contains three steps. For An(III)+RE recovery process, we developed TDdDGA which has very high performance to recover of MA from high level waste. HONTA and ADAAM were developed for An(III)/RE separation process and Am/Cm separation process respectively. All extractants satisfy CHON principle to minimization of the secondary waste from the process. The separation performances of the flowsheets were evaluated by continuous extraction tests using simulated and genuine high level liquid waste.
Suzuki, Hideya*; Shimojo, Kojiro; Nakamura, Satoshi; Emori, Tatsuya; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; Kawasaki, Tomohiro*; Ban, Yasutoshi
no journal, ,
The Japan Atomic Energy Agency (JAEA) has been studying partitioning and transmutation (P&T) systems. In P&T, JAEA proposed a novel hydrometallurgical process called "SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process" for nuclear fuel reprocessing and minor actinides (MA) separation. In this study, a new MA separation method was investigated to further improve the efficiency of the SELECT process. Batch tests were conducted in a mixed solvent system using the acidic amide-type extractants nitrilotriacetic acid diacetamide with branched side chains (B-TONAADA) and alkyl diamidoamine (ADAAM). As a result, we found an efficient method for separating only americium (Am) from an aqueous nitric acid solution containing MA and rare earth elements by adjusting nitric acid concentration.
Okada, Makoto*; Watanabe, So; Nakase, Masahiko*; Ban, Yasutoshi; Shiwaku, Hideaki; Matsuura, Haruaki*
no journal, ,
no abstracts in English
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Toigawa, Tomohiro; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; et al.
no journal, ,
PUREX process was established for industrial scale reprocessing plant. TRUEX and the 4 group separation were developed for partitioning of minor actinides from HLW, and demonstrated using genuine HLW. Although the extractants for the processes have excellent performance, the molecules contain phosphorus which could be cause for the secondary waste from the solvent extraction processes. To minimize the radioactive waste, we have conducted research and development of the new reprocessing and MA separation processes using innovative extractants in accord with CHON principle. The extractants for reprocessing process are monoamides as alternative extractants for TBP. For An(III)+RE recovery process, we developed TDdDGA. HONTA and ADAAM were developed for An(III)/RE separation process and Am/Cm separation process respectively. The separation performances of the flowsheets were evaluated by continuous extraction tests using simulated and genuine spent fuel and high level liquid waste.