Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Iwamori, Akiyuki*; Ogita, Yasuhiro; Shimada, Koji; Tateishi, Ryo*; Takagi, Hideo*; Ota, Toru*; Cho, T.*; Kudo, Shunsuke*; Nojiri, Keisuke*; Shigemitsu, Yasumune*; et al.
Engineering Geology, 344, p.107821_1 - 107821_20, 2025/01
Times Cited Count:2 Percentile:39.43(Engineering, Geological)Clarification of the physicochemical characterization of brittle fault rocks is important not only for understanding the history of the fault activity and deformation mechanisms, but also for assessing the siting conditions of important facilities such as nuclear power plants, radioactive waste disposal sites, and oil storage bases. Here, we apply the chemical weathering index (W values) to the brittle fault rocks of the Shiraki-Nyu fault (granite), the Tsuruga fault (geological boundary between granite and greenstone), and the Yamada fault (adamellite), which are active faults in the peripheral area of Wakasa Bay, and their respective protoliths (hard rocks), and investigate the physicochemical characteristics of the youngest active domain of brittle fault rocks based on the relationship between computed tomography data (CT numbers) and alteration intensity (AI values). The W values of the fault rocks are mainly affected by changes in Na O and CaO, corresponding to the elution or deposition of plagioclase and calsite for granite, clinopyroxene and hornblende for greenstone, and plagioclase for adamellite. The W values mainly indicate the effects of hydrothermal alteration up to 50
O and CaO, corresponding to the elution or deposition of plagioclase and calsite for granite, clinopyroxene and hornblende for greenstone, and plagioclase for adamellite. The W values mainly indicate the effects of hydrothermal alteration up to 50  60 percent and of weathering at over 60 percent. On the other hand, the CT values of the fault rocks are lowest in the fault gouge corresponding to the latest active zone, which was identified as the lowest density zone. In addition, fresh plagioclase fragments are present in each fault gouge of the latest active zone of the active faults in this study. The application of W values to brittle fault rocks is an effective method for understanding the trends of mineralogical variations associated with hydrothermal alteration and weathering in fault rocks, and it is highly possible to improve the accuracy of identifying the youngest active domain in major fault zones through joint analyses of CT numbers.
60 percent and of weathering at over 60 percent. On the other hand, the CT values of the fault rocks are lowest in the fault gouge corresponding to the latest active zone, which was identified as the lowest density zone. In addition, fresh plagioclase fragments are present in each fault gouge of the latest active zone of the active faults in this study. The application of W values to brittle fault rocks is an effective method for understanding the trends of mineralogical variations associated with hydrothermal alteration and weathering in fault rocks, and it is highly possible to improve the accuracy of identifying the youngest active domain in major fault zones through joint analyses of CT numbers.
Collaborative Laboratories for Advanced Decommissioning Science; Hokkaido University*
JAEA-Review 2021-070, 98 Pages, 2022/03
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2020. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2020, this report summarizes the research results of the "Study on rational treatment/disposal of contaminated concrete waste considering leaching alteration" conducted in FY2020. The present study aims to understand migration behaviors of radionuclides in relation to the properties of concrete materials altered due to leaching, to develop a model to simulate the migration behaviors based on the experimental findings, and to analyze waste management scenarios for radioactive concrete. The focus of the study is the underground concrete structures of Fukushima Daiichi Nuclear Power Station, which is in contact with contaminated water.
Wilson, J.*; Bateman, K.; Tachi, Yukio
Applied Geochemistry, 130, p.104979_1 - 104979_19, 2021/07
Times Cited Count:22 Percentile:53.51(Geochemistry & Geophysics)The concept of deep geological disposal will include the multiple use of cement-based materials. In the case of argillaceous host rocks, the presence of hyperalkaline cement porefluid results in the destabilization of primary minerals in the argillite, resulting in the development of a zone of alteration at cement-rock interfaces. The process understanding gained from experimental, analogue, and modelling studies has been reviewed, and remaining areas of uncertainty identified. Although there is a reasonably good understanding of the mineral assemblages that are likely to occur due to cement-rock interactions, there are still some areas where a degree of uncertainty remains, in particular: the evolution of cement-argillite interfaces at T  25
25 C; the rates at which secondary minerals form; the extent of pore clogging due to secondary mineral precipitation; the implications of alteration for radionuclide transport.
C; the rates at which secondary minerals form; the extent of pore clogging due to secondary mineral precipitation; the implications of alteration for radionuclide transport.
Niwa, Masakazu; Ueki, Tadamasa*; Hoshi, Hiroyuki*; Sugisaki, Yuichi*
JAEA-Research 2020-003, 33 Pages, 2020/07
Ages of volcanic rocks are helpful information to understand the impact of volcanism concerning a site characterization and a safety assessment for geological disposal. In this study, mineralogical and geochemical data of altered volcanic rocks were collected using a polarizing microscope, X-ray diffractometer, X-ray fluorescence spectrometer, X-ray analytical microscope, and electron probe microanalyzer, to select targets suitable for reliable K-Ar dating. In addition, sample preparation procedures such as freeze-thawing and HCl treatment were examined to concentrate unaltered plagioclase which is one of major phenocrysts in volcanic rocks. These data and procedures were compiled in this report.
Takahatake, Yoko; Shibata, Atsuhiro; Nomura, Kazunori; Sato, Tsutomu*
Minerals (Internet), 7(12), p.247_1 - 247_13, 2017/12
Times Cited Count:9 Percentile:40.38(Geochemistry & Geophysics)Hydrous sodium titanate (SrTreat) is able to remove radioactive Sr from Radioactive contaminated water at Fukushima Daiichi Nuclear Power station (F1NPS). Knowing the amount of radioactive nuclides in the used SrTreat is important for an effective disposal and deposition of the F1NPS waste. This study investigated changes in the ability of SrTreat to sorb Sr during its use, and to understand the causes of changes in the sorbing. After exposure to a simulated treated water for 99 h, the surface structure of the SrTreat was changed, and the percentage of sorbed Sr and the buffer capacity for protons decreased. When the amount of radioactive nuclides contained in the used SrTreat is calculated from the sorption data of the as received SrTreat.
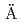 sp
sp Laboratory, Sweden
Laboratory, SwedenSasamoto, Hiroshi; Isogai, Takeshi*; Kikuchi, Hirohito*; Sato, Hisao*; Svensson, D.*
Clay Minerals, 52(1), p.127 - 141, 2017/03
Times Cited Count:5 Percentile:14.48(Chemistry, Physical)Compacted bentonite has been considered as a candidate of engineering barrier material in many countries for the safe disposal of high-level radioactive waste. SKB set up an in situ experiment (named ABM project) to compare the stability of different bentonites under the conditions exposed to an iron source and elevated temperature (up to 130 C as maximum) at the
C as maximum) at the 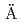 sp
sp Hard Rock Laboratory, Sweden. Results for the Japanese bentonite (Kunigel V1) are summarized in the present paper. Mineralogical investigation using X-ray diffraction (XRD) and X-ray spectroscopy (SEM-EDX) suggested that no indication of smectite transformation or newly formed clay phases were observed. However, a distinct change of exchangeable cations of smectite was indicated (i.e., from Na type to Fe type) in the bentonite at the vicinity of the steel heater. Physical investigation by measurements of hydraulic conductivity and swelling property suggested that no significant change occur in the bentonite even at the vicinity of the steel heater. Such results might be considered due to the limited portion affected by the iron-bentonite interactions and partially occurred ion exchange reactions. Chemical investigation based on the measurements of methylane blue (MB), cation exchange capacity (CEC) and exchangeable cations showed that the lateral distribution for these parameters were basically constant without the significant gradient.
Hard Rock Laboratory, Sweden. Results for the Japanese bentonite (Kunigel V1) are summarized in the present paper. Mineralogical investigation using X-ray diffraction (XRD) and X-ray spectroscopy (SEM-EDX) suggested that no indication of smectite transformation or newly formed clay phases were observed. However, a distinct change of exchangeable cations of smectite was indicated (i.e., from Na type to Fe type) in the bentonite at the vicinity of the steel heater. Physical investigation by measurements of hydraulic conductivity and swelling property suggested that no significant change occur in the bentonite even at the vicinity of the steel heater. Such results might be considered due to the limited portion affected by the iron-bentonite interactions and partially occurred ion exchange reactions. Chemical investigation based on the measurements of methylane blue (MB), cation exchange capacity (CEC) and exchangeable cations showed that the lateral distribution for these parameters were basically constant without the significant gradient.
Yamaguchi, Tetsuji; Sawaguchi, Takuma; Tsukada, Manabu; Hoshino, Seiichi*; Tanaka, Tadao
Clay Minerals, 51(2), p.279 - 287, 2016/02
Times Cited Count:10 Percentile:29.21(Chemistry, Physical)Alteration of bentonite-cement interfaces and accompanying changes in diffusivity of tritiated water was experimentally investigated using intact hardened cement specimens. The alteration by carbonate solution was accompanied by mineralogical changes at the interface and a decrease in the diffusivity to 70% of the initial value after 180-day period. Another alteration under silicate system contacting hardened cement and compacted bentonite was accompanied by mineralogical changes at the interface and a decrease in the diffusivity to 71% of the initial value after 600-day period. The changes in the diffusivity were much less than those observed for mixed specimens of granulated hardened cement and bentonite where the diffusivity decreased down to 20% of the initial value over 180 days. The results were extrapolated to 15 years under simple assumptions and showed good agreement with those observed in the cement-argillite interface at Tournemire URL. Such an explanation enhances our confidence in our assessment of alteration of cement-bentonite systems and can be a base for using our data and models in long term assessment of radioactive waste disposal.
Yuguchi, Takashi; Sasao, Eiji; Ishibashi, Masayuki; Nishiyama, Tadao*
American Mineralogist, 100(5-6), p.1134 - 1152, 2015/05
Times Cited Count:40 Percentile:75.69(Geochemistry & Geophysics)This paper describes the biotite chloritization process with a focus on mass transfer in the Toki granitic pluton, Central Japan, and also depicts the temporal variations in chemical characteristics of hydrothermal fluid associated with chloritization during the sub-solidus cooling of the pluton. Singular value decomposition (SVD) analysis results in chloritization reaction equations for eight mineral assemblages, leading to the quantitative assessment of mass transfer between the reactant and product minerals, and inflow and outflow of components through the hydrothermal fluid. The matrices for SVD analysis consist of arbitrary combinations of molar volume and closure component in the reactant and product minerals. The eight reactions represent the temporal variations of chemical characteristics of the hydrothermal fluid associated with chloritization: the progress of chloritization results in gradual increase of silicon, potassium and chlorine and gradual decrease of calcium and sodium in the hydrothermal fluid with temperature decrease. The biotite chloritization involves two essential formation processes: Formation Process 1, small volume decrease from biotite to chlorite and large inflow of metallic ions from the hydrothermal fluid, and Formation Process 2, large volume decrease and large outflow of metallic ions into hydrothermal fluid. Chlorite produced during Formation Process 1 dominates over that of Formation Process 2, resulting in the gradual decrease of metallic components in the hydrothermal fluid with chloritization progress. The combination of continuous reactions based on compositional variations in chlorite together with corresponding continuous Al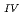 variations gives an indication of the temporal variations in rates of decreasing and increasing concentration of chemical components in the hydrothermal fluid associated with chloritization.
variations gives an indication of the temporal variations in rates of decreasing and increasing concentration of chemical components in the hydrothermal fluid associated with chloritization.
Yamaguchi, Tetsuji; Sakamoto, Yoshifumi; Akai, Masanobu; Takazawa, Mayumi; Iida, Yoshihisa; Tanaka, Tadao; Nakayama, Shinichi
Physics and Chemistry of the Earth, 32(1-7), p.298 - 310, 2007/00
Times Cited Count:47 Percentile:73.53(Geosciences, Multidisciplinary)Dissolution rate of montmorillonite, diffusivity of hydroxide ion and permeability coefficient in compacted sand-bentonite mixtures were experimentally determined and formulated. A coupled mass-transport/chemical-reaction code was developed to predict variation in permeability of engineered bentonite barrier with alkaline fluid by using the formulae.
Nakayama, Shinichi; Sakamoto, Yoshifumi; Yamaguchi, Tetsuji; Akai, Masanobu; Tanaka, Tadao; Sato, Tsutomu*; Iida, Yoshihisa
Applied Clay Science, 27(1-2), p.53 - 65, 2004/10
Times Cited Count:91 Percentile:90.27(Chemistry, Physical)Alkaline environments induced by cement in radioactive waste repositories are likely to alter montmorillonite, the main constituent of bentonite buffer materials. Over long time periods, the alteration may cause the physical and/or chemical properties of the buffer to deteriorate. For the purpose of acquiring numerical data to quantify the effect of alteration on permeability of bentonite buffer, dissolution rates of montmorillonite and diffusivity of hydroxide ions in compacted sand-bentonite mixture specimens have been measured under highly alkaline, simulated groundwater conditions. The dissolution rate of montmorillonite was given by the linear dependence on time under the employed experimental conditions of pH 13 to 14 and temperatures of 90 to 170 C. The diffusivity of hydroxide ions was obtained in through-diffusion experiments combined with a pore diffusion model. The experiments were performed under relatively low temperatures of 10 to 50
C. The diffusivity of hydroxide ions was obtained in through-diffusion experiments combined with a pore diffusion model. The experiments were performed under relatively low temperatures of 10 to 50 C to minimize the effect of alteration of bentonite. The effective diffusivity was on the order of 10
C to minimize the effect of alteration of bentonite. The effective diffusivity was on the order of 10 to 10
to 10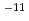 m
m /s.
/s.
 -montmorillonite
-montmorilloniteKawakita, Ryohei*; Saito, Akito*; Sakuma, Hiroshi*; Anraku, Sohtaro; Oda, Chie; Mihara, Morihiro; Sato, Tsutomu*
no journal, ,
no abstracts in English
Yuguchi, Takashi; Sasao, Eiji; Ishibashi, Masayuki; Nishiyama, Tadao*
no journal, ,
no abstracts in English
Bateman, K.; Amano, Yuki; Tachi, Yukio
no journal, ,