Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kinoshita, Ryoma; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Hydrometallurgy, 222, p.106159_1 - 106159_12, 2023/10
Times Cited Count:2 Percentile:23.52(Metallurgy & Metallurgical Engineering)Solvent extraction is conducted using a total of 20 metals revealing high stability constants with Cl and hexahexyl-nitrilotriacetamide (NTAamide(C6)) extractant. The metals used here may behave as anions at high Cl concentrations, and NTAamide(C6), which contains a tertiary N atom, is protonated under acidic conditions. Most of the metal ions in this study display higher distribution ratios (D(M)) from HCl than those from HNO , and exhibit 1:1 stoichiometries with NTAamide. Following the experimental results, the association constants and distribution coefficients of the group 12 elements are calculated via ion-pair extraction modeling using density functional theory calculations, and the simulations of D yield calculated values with the same trend as that of the measured values.
, and exhibit 1:1 stoichiometries with NTAamide. Following the experimental results, the association constants and distribution coefficients of the group 12 elements are calculated via ion-pair extraction modeling using density functional theory calculations, and the simulations of D yield calculated values with the same trend as that of the measured values.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:3 Percentile:35.90(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
Iwamoto, Toshihiro; Saito, Madoka*; Takahatake, Yoko; Watanabe, So; Watanabe, Masayuki; Naruse, Atsuki*; Tsukahara, Takehiko*
Proceedings of 30th International Conference on Nuclear Engineering (ICONE30) (Internet), 4 Pages, 2023/05
Applicability of temperature swing extraction technology employing monoamides was examined for uranium contaminated waste treatment procedure. Separation experiments on simulated target solution with three kinds of monoamides with different structure showed that Ce(IV) in the solution was selectively recovered by the temperature swing extraction operation. Based on the experiments, an appropriate monoamide for the procedure was selected.
Yamagata, Kazuhito*; Ouchi, Kazuki; Marumo, Kazuki*; Tasaki-Handa, Yuiko*; Haraga, Tomoko; Saito, Shingo*
Inorganic Chemistry, 62(2), p.730 - 738, 2023/01
Times Cited Count:3 Percentile:40.57(Chemistry, Inorganic & Nuclear)The inert NpO
 complex with a fluorescein-modified phenanthroline-2,9-dicarboxylic acid was found by kinetic selection using polyacrylamide gel electrophoresis (PAGE) from a small chemical library. The small spontaneous dissociation rate constant of 8
complex with a fluorescein-modified phenanthroline-2,9-dicarboxylic acid was found by kinetic selection using polyacrylamide gel electrophoresis (PAGE) from a small chemical library. The small spontaneous dissociation rate constant of 8 10
10 s
s (the half-life of 23 hours) was determined. This is the singly-charged NpO
(the half-life of 23 hours) was determined. This is the singly-charged NpO
 complex exhibiting unusual kinetic inertness in aqueous solution, one million times slower than widely accepted fast kinetics of neptunyl complexes. Selective fluorescence detection of NpO
complex exhibiting unusual kinetic inertness in aqueous solution, one million times slower than widely accepted fast kinetics of neptunyl complexes. Selective fluorescence detection of NpO
 was achieved in PAGE with a detection limit of 68 pmol dm
was achieved in PAGE with a detection limit of 68 pmol dm (17 fg). This system was successfully applied to simulated spent nuclear fuel and high-level radioactive waste samples.
(17 fg). This system was successfully applied to simulated spent nuclear fuel and high-level radioactive waste samples.
Simonnet, M.; Sasaki, Yuji; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 41(7), p.857 - 867, 2023/00
Times Cited Count:4 Percentile:41.44(Chemistry, Multidisciplinary)Sasaki, Yuji; Morita, Keisuke; Ito, Keisuke; Suzuki, Shinichi; Shiwaku, Hideaki; Takahashi, Yuya*; Kaneko, Masaaki*; Omori, Takashi*; Asano, Kazuhito*
Proceedings of International Nuclear Fuel Cycle Conference (GLOBAL 2017) (USB Flash Drive), 4 Pages, 2017/09
no abstracts in English
Sasaki, Yuji; Yoshimitsu, Ryo*; Nishihama, Shohei*; Shimbori, Yuma*; Shiroishi, Hidenobu*
Separation Science and Technology, 52(7), p.1186 - 1192, 2017/03
Times Cited Count:2 Percentile:8.14(Chemistry, Multidisciplinary)The new extractant, biuret(C8), is synthesized and tested for the solvent extraction of hard acid metals like actinides and soft acid metals. This compound has the similar central frame to malonamide but with 2-NH introduced into the central frame, then both amidic oxygen and nitrogen atoms may bond with metals. From the present work, not only hard acid metals, but also soft acid metals can be extracted by biuret(C8) from nitric or perchloric acids to n-dodecane. The extractability for biuret(C8) is compared with other representative extractants, malonamide, TODGA and MIDOA. It is clear that the distribution ratio(D) of U and Pu by biuret(C8) is similar to those for malonamide, but with lower values than those for TODGA and MIDOA, and D for soft acid metals and oxonium anions show higher values than those for TODGA and malonamide and lower than those for MIDOA.
 ,
, -dialkylamides-nitric acid systems
-dialkylamides-nitric acid systemsTsutsui, Nao; Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Urabe, Shunichi; Matsumura, Tatsuro
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1153 - 1157, 2015/09
 ,
, -Dialkylamides are promising alternative extractants to tri-
-Dialkylamides are promising alternative extractants to tri- -butyl phosphate in the reprocessing of spent nuclear fuels, but the two-phase separation between their organic and aqueous phases has not been evaluated quantitatively.
-butyl phosphate in the reprocessing of spent nuclear fuels, but the two-phase separation between their organic and aqueous phases has not been evaluated quantitatively.  ,
, -Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) in
-Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) in  -dodecane were agitated with uranyl nitrate-containing nitric acid, and their turbidities and their uranium distribution ratios were measured with respect to the time for the quantitative evaluation. Increasing DEHDMPA, uranium, and nitric acid concentrations enhanced turbidities. Although turbidities decreased with respect to the time, uranium distribution ratios slightly changed, indicating the observed turbidities did not affect these uranium distribution ratios significantly. Therefore, DEHDMPA may act as suitable extractant for uranium in nitric acid from two-phase separation viewpoint, and turbidity may be an indicator for extractant performance evaluation.
-dodecane were agitated with uranyl nitrate-containing nitric acid, and their turbidities and their uranium distribution ratios were measured with respect to the time for the quantitative evaluation. Increasing DEHDMPA, uranium, and nitric acid concentrations enhanced turbidities. Although turbidities decreased with respect to the time, uranium distribution ratios slightly changed, indicating the observed turbidities did not affect these uranium distribution ratios significantly. Therefore, DEHDMPA may act as suitable extractant for uranium in nitric acid from two-phase separation viewpoint, and turbidity may be an indicator for extractant performance evaluation.
Sasaki, Yuji; Suzuki, Hideya*; Sugo, Yumi; Kimura, Takaumi; Choppin, G. R.*
Chemistry Letters, 35(3), p.256 - 257, 2006/03
Times Cited Count:40 Percentile:69.16(Chemistry, Multidisciplinary)Novel water-soluble organic ligands, N,N,N',N'-tetramethyl-diglycolamide (TMDGA), N,N,N',N'-tetraethyl-diglycolamide (TEDGA), N,N,N',N'-tetrapropyl-diglycolamide (TPDGA) and N,N-dipropyl-diglycolamic acid (DPDGAc), were synthesized and examined for complexation with An(III) and An(IV). These reagents are readily soluble in water and form more stable complexes with Pu(IV) and Am(III) than does EDTA of hexadentate ligand. TEDGA and TPDGA can back-extract effectively An into the aqueous phase. In addition, the An-complex in the aqueous phase can be dissociated and the TEDGA can be separated from the An and recovered by extraction using a polar organic solvent.
Ikeura, Hiromi*; Sekiguchi, Tetsuhiro; Baba, Yuji; Imamura, Motoyasu*; Matsubayashi, Nobuyuki*; Shimada, Hiromichi*
Surface Science, 593(1-3), p.303 - 309, 2005/11
Times Cited Count:5 Percentile:25.21(Chemistry, Physical)no abstracts in English
 -dodecane containing a solvent modifier
-dodecane containing a solvent modifierSasaki, Yuji; Zhu, Z.-X.; Sugo, Yumi; Suzuki, Hideya*; Kimura, Takaumi
Analytical Sciences, 21(10), p.1171 - 1175, 2005/10
Times Cited Count:30 Percentile:62.22(Chemistry, Analytical)In order to evaluate the extraction property of the new extractants, diglycolamide(DGA) compounds, we investigated the maximum extraction of di-, tri-, and tetravalent metal ions using the system of nitric acid and n-dodecane. Limits of metal concentration (LOC) for Ca(II), Nd(III) and Zr(IV) influence strongly on HNO and the extractant concentration. On the purpose to raise the LOC value, we employed the modifier of solvent, N,N-dihexyl-octanamide(DHOA) and DGA with the long alkyl chain, and examined. It is evident that LOC increases with DHOA concentration in n-dodecane and the length of alkyl chain attached with N atom of DGA. The stoichiometric values of LOC(Zr) estimated from the extraction reaction is obtained by using the extraction condition: tetraoctyl-DGA/ 1M DHOA+n-dodecane and 3M HNO
and the extractant concentration. On the purpose to raise the LOC value, we employed the modifier of solvent, N,N-dihexyl-octanamide(DHOA) and DGA with the long alkyl chain, and examined. It is evident that LOC increases with DHOA concentration in n-dodecane and the length of alkyl chain attached with N atom of DGA. The stoichiometric values of LOC(Zr) estimated from the extraction reaction is obtained by using the extraction condition: tetraoctyl-DGA/ 1M DHOA+n-dodecane and 3M HNO .
.
Sasaki, Yuji; Zhu, Z.-X.; Sugo, Yumi; Kimura, Takaumi
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 5 Pages, 2005/10
The extraction of metals by N,N,N',N'-tetraoctyl-diglycolamide (TODGA) from nitric acid to n-dodecane was investigated. From the measurement of distribution ratios, it was obvious that Ca (ionic radius: 100pm), trivalent and tetravalent ions with ionic radii of 87-113 and 83-94 pm are highly extractable by TODGA. In order to evaluate the extraction capacity in extraction solvent using DGA compounds, we measured the limits of metal concentration (LOC) for Ca(II), Nd(III) and Zr(IV). For the purpose to enhance the LOC value, we examined the modifier of solvent, N,N-dihexyl-octanamide(DHOA) and DGA with longer alkyl chain. It is evident that LOC is increased with DHOA concentration and the length of alkyl chain attached to N atom of DGA.
 -tetraalkylderivatives of diglycolamide-monoamide/n-dodecane media
-tetraalkylderivatives of diglycolamide-monoamide/n-dodecane mediaSasaki, Yuji; Sugo, Yumi; Suzuki, Shinichi; Kimura, Takaumi
Analytica Chimica Acta, 543(1-2), p.31 - 37, 2005/07
Times Cited Count:116 Percentile:94.65(Chemistry, Analytical)The detailed study of extraction capacity of diglycolamide (DGA) with and without DHOA, N,N'-dihexyloctanamide as a modifier, was performed by using the novel analytical method for the determination of the limit of metal concentration in the organic phase (LOC). The effect on modifier was studied and it is confirmed that LOC increases with DHOA concentration. It is noteworthy that LOC reaches to the stoichiometric value, which is obtained by the extraction reaction, by using 0.1M TODGA mixed with more than 0.5M DHOA into n-dodecane. Without modifier, relation of the different DGA compounds with LOC was investigated, and it is clear that LOC using N,N,N',N'-tetradodecyl-diglycolamide (TDdDGA), whose extractant can suppress the third phase, reaches to the stoichiometric value. From this work, it is obvious that the application of the condition without the third phase formation, e.g., enough concentration of modifier coexisted and DGA with long alkyl chain, is important to attain the stochiometric LOC value.
Kato, Jun*; Maekawa, Yasunari; Yoshida, Masaru
Chemistry Letters, 34(2), p.266 - 267, 2005/02
Times Cited Count:12 Percentile:42.51(Chemistry, Multidisciplinary)We have investigated the useful EB induced reactions of sulfonamide and sulfonate derivatives in the crystalline state. In this letter, we found that the EB sensitivity of sulfonic acid derivatives in the crystalline tate was much higher than that of the corresponding carboxylic acid derivatives, which was distinct from the results using other energy sources, to give Fries rearrangement products. It is notable that these reactions are solvent-free and are accompanied by the transformation of amide and ester linkages to hydrophilic aniline and phenol groups; especially, an acidic sulfonamide can be converted to the corresponding basic aniline derivatives. These new EB induced transformations can significantly contribute to designing new materials for EB lithography as well as nanoscopic architectures.
 -tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into
-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into  -dodecane
-dodecaneZhu, Z.-X.; Sasaki, Yuji; Suzuki, Hideya*; Suzuki, Shinichi; Kimura, Takaumi
Analytica Chimica Acta, 527(2), p.163 - 168, 2004/12
Times Cited Count:286 Percentile:99.20(Chemistry, Analytical)The extraction of 75 elements by  -tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid to
-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid to  -dodecane was investigated and discussed concerning distribution ratios (D) of metal ions and their ionic radii. The extractability for elements depends strongly on their oxidation state. Monovalent and pentavalent ions were not extractable. In divalent metal extraction, Ca(II) (ionic radius: 100 pm) showed the highest D value and the D decreased with increase or decrease of the ionic radius. Tri- and tetravalent ions with ionic radii of 87-113 pm and 83-94 pm, respectively, showed the D over 1000, and the extracted chemical forms were determined to be M(TODGA)
-dodecane was investigated and discussed concerning distribution ratios (D) of metal ions and their ionic radii. The extractability for elements depends strongly on their oxidation state. Monovalent and pentavalent ions were not extractable. In divalent metal extraction, Ca(II) (ionic radius: 100 pm) showed the highest D value and the D decreased with increase or decrease of the ionic radius. Tri- and tetravalent ions with ionic radii of 87-113 pm and 83-94 pm, respectively, showed the D over 1000, and the extracted chemical forms were determined to be M(TODGA) (NO
(NO )
)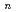 or M(TODGA)
or M(TODGA) (NO
(NO )
)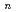 (
( =3 or 4). The oxonium ions with higher oxidation states than tetravalent one forms the complicated metal-complex with TODGA, and the number of TODGA associated in those extraction reactions was confirmed to be 2 at most.
=3 or 4). The oxonium ions with higher oxidation states than tetravalent one forms the complicated metal-complex with TODGA, and the number of TODGA associated in those extraction reactions was confirmed to be 2 at most.
Suzuki, Shinichi; Sasaki, Yuji; Yaita, Tsuyoshi; Kimura, Takaumi
Proceedings of International Conference ATALANTE 2004 Advances for Future Nuclear Fuel Cycles (CD-ROM), 4 Pages, 2004/06
An innovative chemical separation process (ARTIST: Amide-based Radio-resources Treatment with Interim Storage of Transuranics) was proposed for the treatment of spent nuclear fuel. The main concept of ARTIST process is to recover and stock all actinides (An) and to dispose the fission products (FP). One of the main purposes of this process is selective isolation of uranium. Since the brached alkyl type N,N-dialkyl-monoamides (BAMA) have the steric hindrance on the complexation with metal cations, BAMA can be used to separate An(VI) from An(IV). N,N-di-(2-ethyl)hexyl-2,2-dimethylpropanamide (D2EHDMPA) can recover U(VI) selectively without accumulating Pu(IV) in uranium isolation process. From extraction behavior of Np, D2EHDMPA can extract and separate U(VI) from Np(VI) without reduction from Np(VI) to Np(V) or Np(IV).
 solutions using
solutions using  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide
-diphenylpyridine-2,6-dicarboxyamideShimada, Asako; Yaita, Tsuyoshi; Narita, Hirokazu; Tachimori, Shoichi; Kimura, Takaumi; Okuno, Kenji*;
Solvent Extraction Research and Development, Japan, 11, p.1 - 10, 2004/04
The distribution ratio ( ) of Am(III) and lighter Ln(III) in the extraction with
) of Am(III) and lighter Ln(III) in the extraction with  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO solutions were determined. The D's increased with an increase of HNO
solutions were determined. The D's increased with an increase of HNO concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO
concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO concentration range (S.F.=(D
concentration range (S.F.=(D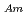 /D
/D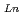 )). From 4 M HNO
)). From 4 M HNO solution with 0.5 M DMDPhPDA CHCl
solution with 0.5 M DMDPhPDA CHCl solution, the
solution, the  's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO
's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO concentration region than that has been reported so far. These
concentration region than that has been reported so far. These  's value and the S.F. were reproduced in the extraction from the metal concentration range from 10
's value and the S.F. were reproduced in the extraction from the metal concentration range from 10 M order to 10
M order to 10 M.
M.
Norisue,Tomohisa*; Kida, Yusuke*; Masui, Naoki*; Tran-Cong-Miyata, Q.*; Maekawa, Yasunari; Yoshida, Masaru; Shibayama, Mitsuhiro*
Macromolecules, 36(16), p.6202 - 6212, 2003/08
Times Cited Count:80 Percentile:89.40(Polymer Science)The shrinking kinetics of poly(N-isopropylacrylamide) (PNIPA) gels has been studied for two types of PNIPA gels prepared by (i) copolymerization of constituent monomer and cross-linker and (ii)  -ray irradiation in the PNIPA solutions in order to investigate the role of cross-linking on shrinking kinetics. The shrinking kinetics of the monomer cross-linked gels is quite similar to that of the polymer cross-linked gels. On the other hand, a significant difference was found when the microscopic structure and the dynamics were investigated by small-angle neutron scattering (SANS) and static/dynamic light scattering (SLS/DLS). The degree of built-in inhomogeneities and dynamic fluctuations were evaluated as a function of the cross-linking degree and the gel preparation temperature by intensity decomposition methods for both types of gels. It is concluded that the monomer cross-linked gels have extra built-in inhomogeneities due to the spatial distribution of crosslinks in addition to the frozen concentration fluctuations inherent in polymer gels.
-ray irradiation in the PNIPA solutions in order to investigate the role of cross-linking on shrinking kinetics. The shrinking kinetics of the monomer cross-linked gels is quite similar to that of the polymer cross-linked gels. On the other hand, a significant difference was found when the microscopic structure and the dynamics were investigated by small-angle neutron scattering (SANS) and static/dynamic light scattering (SLS/DLS). The degree of built-in inhomogeneities and dynamic fluctuations were evaluated as a function of the cross-linking degree and the gel preparation temperature by intensity decomposition methods for both types of gels. It is concluded that the monomer cross-linked gels have extra built-in inhomogeneities due to the spatial distribution of crosslinks in addition to the frozen concentration fluctuations inherent in polymer gels.
Sekiguchi, Tetsuhiro; Ikeura, Hiromi*; Baba, Yuji
Surface Science, 532-535(1-3), p.1079 - 1084, 2003/06
Using a newly developed rotatable time-of-flight mass spectrometer(R-TOF-MS) and polarized synchrotron radiation, we have investigated orientation effect on fragmentation and desorption pathways occurring at the top-most layers of molecular solids. Reported will be polarization-angle dependencies of TOF mass spectra, high-resolution electron- and ion-NEXAFS in condensed formic acid, formamide and benzene. For condensed formamide(HCOND ), marked orientation effect was observed for the enhanced H
), marked orientation effect was observed for the enhanced H -yields following C1s
-yields following C1s 
 *
*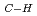 resonance. Direct photodissociation and charge-neutralization play an important role in the effect. For some fragment species, however, the bond scission showed no polarization dependence when dissociation sites were far from core-excited atoms. This is the case for N-D scission and D
resonance. Direct photodissociation and charge-neutralization play an important role in the effect. For some fragment species, however, the bond scission showed no polarization dependence when dissociation sites were far from core-excited atoms. This is the case for N-D scission and D -desorption following C1s excitation, suggesting that indirect process governs, where secondary electrons would induce the fragmentation.
-desorption following C1s excitation, suggesting that indirect process governs, where secondary electrons would induce the fragmentation.
Tachimori, Shoichi
JAERI-Research 2001-048, 23 Pages, 2001/10
A new chemical process, ARTIST process, is proposed for the treatment of spent nuclear fuel. The main concept of the ARTIST process is to recover and stock all actinides (Ans) in two groups, uranium (U) and a mixture of transuranics (TRU), to preserve their resource value and to dispose solely fission products (FPs). The process composed of two main steps, an U exclusive isolation and a total recovery of TRU; which copes with the nuclear non-proliferation measures, and additionally Pu separation process and soft N-donor process if requested, and optionally processes for separation of long-lived FPs. These An products: U-product and TRU-product, are to be solidified by calcination and allowed to the interim stockpile for future utilization. These separations are achieved by use of amidic extractants in accord with the CHON principle. The technical feasibility of the ARTIST process was explained by the performance of both the branched-alkyl monoamides the diglycolic amide (TODGA) in thorough extraction of all TRU by tridentate fashon.