Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Mori, Airi; Johansen, M. P.*; McGinnity, P.*; Takahara, Shogo
Communications Earth & Environment (Internet), 6, p.356_1 - 356_11, 2025/05
Times Cited Count:0 Percentile:0.00(Environmental Sciences) Cs contamination of Japanese mustard spinach by resuspended particles in areas with different contamination conditions
Cs contamination of Japanese mustard spinach by resuspended particles in areas with different contamination conditionsTatsuno, Takahiro*; Yoshimura, Kazuya; Nihei, Naoto*
Journal of Radioanalytical and Nuclear Chemistry, 333(3), p.1089 - 1096, 2024/03
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)Kim, M.; Malins, A.*; Machida, Masahiko; Yoshimura, Kazuya; Saito, Kimiaki; Yoshida, Hiroko*
Nihon Genshiryoku Gakkai Wabun Rombunshi (Internet), 22(4), p.156 - 169, 2023/11
Dose reduction factor of a Japanese house is important information in the external exposure estimation of returning residents. In 2019, a total of 19 wooden houses were surveyed in Iitate Village and Namie Town using a gamma plotter that can continuously measure the air dose rate. In addition, the characteristics of the reduction factor were investigated from the measured air dose rate. In the vicinity of houses, uncontaminated areas exist underneath houses and, the ratio of paved surfaces such as asphalt roads is relatively high; furthermore, the pavement has a tendency for the radiation source to decay quickly. Therefore, the air dose rate near the house showed a relatively low value in common at all sites. Air dose rates above unpaved surfaces showed higher values and larger variations than those above paved surfaces within a radius of 50 m form the center of a house. The reduction factor was widely distributed even for one house, if the ratio of every air dose rate observed inside and outside the house is considered. It is suggested that a realistic reduction factor may not be obtained when the reduction factor is obtained based on the measured values at a small number of points that do not have the representativeness of the radiation field to be measured.
 Cs supply from rivers to coastal waters off Fukushima considering human activities
Cs supply from rivers to coastal waters off Fukushima considering human activitiesIkenoue, Tsubasa; Shimadera, Hikari*; Nakanishi, Takahiro; Kondo, Akira*
Water (Internet), 15(15), p.2734_1 - 2734_18, 2023/08
Times Cited Count:2 Percentile:11.44(Environmental Sciences)The Fukushima Daiichi Nuclear Power Plant accident caused an accumulation of  Cs in coastal sediment. The
Cs in coastal sediment. The  Cs supply from rivers to the ocean can affect the long-term fate of
Cs supply from rivers to the ocean can affect the long-term fate of  Cs in coastal sediment. Since the Fukushima coastal river basins include large decontaminated and evacuation order areas, considering the decontamination work and resumption of agriculture is important for predicting the
Cs in coastal sediment. Since the Fukushima coastal river basins include large decontaminated and evacuation order areas, considering the decontamination work and resumption of agriculture is important for predicting the  Cs supply. We conducted a 30-year prediction of the
Cs supply. We conducted a 30-year prediction of the  Cs supply from the Fukushima coastal rivers to the ocean using a distributed radiocesium prediction model, considering the effects of human activities. In river basins with decontaminated and evacuation order areas, human activities reduced the total
Cs supply from the Fukushima coastal rivers to the ocean using a distributed radiocesium prediction model, considering the effects of human activities. In river basins with decontaminated and evacuation order areas, human activities reduced the total  Cs outflow from agricultural lands, urban lands, and forest areas to the rivers and the
Cs outflow from agricultural lands, urban lands, and forest areas to the rivers and the  Cs supply to the ocean by 5.0% and 6.0%, respectively. These results indicated that human activities slightly impacted the
Cs supply to the ocean by 5.0% and 6.0%, respectively. These results indicated that human activities slightly impacted the  Cs outflow and supply. The
Cs outflow and supply. The  Cs supply from rivers impacted by the accident to the coastal sediment was estimated to correspond to 11-36% of the total
Cs supply from rivers impacted by the accident to the coastal sediment was estimated to correspond to 11-36% of the total  Cs in the coastal sediment in the early phase of the accident. Therefore, the
Cs in the coastal sediment in the early phase of the accident. Therefore, the  Cs supply from rivers to the ocean is important for the long-term behavior of
Cs supply from rivers to the ocean is important for the long-term behavior of  Cs in coastal sediment.
Cs in coastal sediment.
 Cs transfer from soils contaminated by resuspended particles to Japanese mustard spinach in difficult-to-return zone of Fukushima
Cs transfer from soils contaminated by resuspended particles to Japanese mustard spinach in difficult-to-return zone of FukushimaTatsuno, Takahiro*; Nihei, Naoto*; Yoshimura, Kazuya; Ote, Nobuhito*
Journal of Radioanalytical and Nuclear Chemistry, 332(6), p.1677 - 1686, 2023/06
Times Cited Count:1 Percentile:23.64(Chemistry, Analytical) Sr/
Sr/ Y and
Y and  Cs radioactivity
Cs radioactivityTerasaka, Yuta; Uritani, Akira*
Nuclear Instruments and Methods in Physics Research A, 1049, p.168071_1 - 168071_7, 2023/04
Times Cited Count:2 Percentile:43.92(Instruments & Instrumentation)Tatsuno, Takahiro*; Waki, Hiromichi*; Kakuma, Minato*; Nihei, Naoto*; Takase, Tsugiko*; Wada, Toshihiro*; Yoshimura, Kazuya; Nakanishi, Takahiro; Ote, Nobuhito*
Journal of Environmental Management, 329, p.116983_1 - 116983_13, 2023/03
Times Cited Count:2 Percentile:11.93(Environmental Sciences)Battulga, B.; Atarashi-Andoh, Mariko; Nakanishi, Takahiro; Koarashi, Jun
Science of the Total Environment, 849, p.157758_1 - 157758_11, 2022/11
Times Cited Count:7 Percentile:45.72(Environmental Sciences)Characterizing plastic-associated biofilms is key to the better understanding of organic material and mineral cycling in the "Plastisphere"-the thin layer of microbial life on plastics. In this study, we propose a new method to extract biofilms from environmental plastics, in order to evaluate the properties of biofilm-derived organic matter through stable carbon (
 C) and nitrogen (
C) and nitrogen (
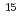 N) isotope signatures and their interactions with radionuclides especially radiocesium (
N) isotope signatures and their interactions with radionuclides especially radiocesium ( Cs). After ultrasound-assisted separation from the plastics, biofilm samples were successfully collected via a sequence of syringe treatments. Biofilm-derived organic matter samples (14.5-65.4 mg) from four river mouths in Japan showed
Cs). After ultrasound-assisted separation from the plastics, biofilm samples were successfully collected via a sequence of syringe treatments. Biofilm-derived organic matter samples (14.5-65.4 mg) from four river mouths in Japan showed  Cs activity concentrations of
Cs activity concentrations of  75 to 820 Bq kg
75 to 820 Bq kg biofilm (dw), providing evidence that environmental plastics, mediated by developed biofilms, serve as a carrier for
biofilm (dw), providing evidence that environmental plastics, mediated by developed biofilms, serve as a carrier for  Cs in the coastal environment. Significant differences in the (
Cs in the coastal environment. Significant differences in the (
 C and
C and 
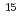 N signatures were also obtained for the biofilms, indicating the different sources, pathways, and development processes of biofilms on plastics.
N signatures were also obtained for the biofilms, indicating the different sources, pathways, and development processes of biofilms on plastics.
Tatsuno, Takahiro*; Waki, Hiromichi*; Kakuma, Minato*; Nihei, Naoto*; Wada, Toshihiro*; Yoshimura, Kazuya; Nakanishi, Takahiro; Ote, Nobuhito*
Radiation Protection Dosimetry, 198(13-15), p.1052 - 1057, 2022/09
Times Cited Count:2 Percentile:28.39(Environmental Sciences) Cs inventory
Cs inventoryYoshimura, Kazuya; Nakama, Shigeo; Fujiwara, Kenso
Journal of Radiation Protection and Research, 47(1), p.30 - 38, 2022/03
Yoshimura, Kazuya
Journal of Nuclear Science and Technology, 59(1), p.25 - 33, 2022/01
Times Cited Count:9 Percentile:70.62(Nuclear Science & Technology) Cs washoff from bare land in Fukushima
Cs washoff from bare land in FukushimaIgarashi, Yasunori*; Onda, Yuichi*; Wakiyama, Yoshifumi*; Yoshimura, Kazuya; Kato, Hiroaki*; Kozuka, Shohei*; Manome, Ryo*
Science of the Total Environment, 769, p.144706_1 - 144706_9, 2021/05
Times Cited Count:4 Percentile:16.79(Environmental Sciences) Cs and
Cs and  Cs radioactivity measurements
Cs radioactivity measurementsMalins, A.; Imamura, Naohiro*; Niizato, Tadafumi; Takahashi, Junko*; Kim, M.; Sakuma, Kazuyuki; Shinomiya, Yoshiki*; Miura, Satoru*; Machida, Masahiko
Journal of Environmental Radioactivity, 226, p.106456_1 - 106456_12, 2021/01
Times Cited Count:7 Percentile:31.11(Environmental Sciences)Malins, A.; Ochi, Kotaro; Machida, Masahiko; Sanada, Yukihisa
Proceedings of Joint International Conference on Supercomputing in Nuclear Applications + Monte Carlo 2020 (SNA + MC 2020), p.147 - 154, 2020/10
 Cs
CsShibahara, Yuji*; Nakamura, Shoji; Uehara, Akihiro*; Fujii, Toshiyuki*; Fukutani, Satoshi*; Kimura, Atsushi; Iwamoto, Osamu
Journal of Radioanalytical and Nuclear Chemistry, 325(1), p.155 - 165, 2020/07
Times Cited Count:12 Percentile:74.60(Chemistry, Analytical)The measurements of isotopic ratios of Cs samples by thermal ionization mass spectrometry were performed for the analysis of their samples used to evaluate nuclear data obtained for  Cs. To obtain a high intensity and stable ion beam, the effects of additive agents on the ionization of Cs were examined. The effect of silicotungstic acid on the ionization of Cs was the largest among the additive agents studied in the present study, while the silicotungstic acid also showed the largest isobaric interference of polyatomic ions. It was demonstrated that as small as 2
Cs. To obtain a high intensity and stable ion beam, the effects of additive agents on the ionization of Cs were examined. The effect of silicotungstic acid on the ionization of Cs was the largest among the additive agents studied in the present study, while the silicotungstic acid also showed the largest isobaric interference of polyatomic ions. It was demonstrated that as small as 2 10
10 g of a Cs sample was sufficient to achieve the analytical precision required to measure the
g of a Cs sample was sufficient to achieve the analytical precision required to measure the  Cs/
Cs/ Cs ratio in the case where an additive agent of TaO/glucose was employed. After examining of the analytical conditions, such as the interference effect due to Ba, the measurements of the isotopic ratios of two Cs samples used in our study using TIMS were conducted, and it was discussed how much the ratios contributed to evaluation of the neutron capture cross-section of
Cs ratio in the case where an additive agent of TaO/glucose was employed. After examining of the analytical conditions, such as the interference effect due to Ba, the measurements of the isotopic ratios of two Cs samples used in our study using TIMS were conducted, and it was discussed how much the ratios contributed to evaluation of the neutron capture cross-section of  Cs.
Cs.
 Cs on paved surfaces affected by the Fukushima Dai-ichi Nuclear Power Plant accident
Cs on paved surfaces affected by the Fukushima Dai-ichi Nuclear Power Plant accidentYoshimura, Kazuya; Watanabe, Takayoshi; Kurikami, Hiroshi
Journal of Environmental Radioactivity, 217, p.106213_1 - 106213_6, 2020/06
Times Cited Count:5 Percentile:18.02(Environmental Sciences)Nakamura, Shoji; Shibahara, Yuji*; Kimura, Atsushi; Iwamoto, Osamu; Uehara, Akihiro*; Fujii, Toshiyuki*
Journal of Nuclear Science and Technology, 57(4), p.388 - 400, 2020/04
Times Cited Count:3 Percentile:25.69(Nuclear Science & Technology)The thermal-neutron capture cross-section (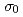 ) and resonance integral(I
) and resonance integral(I ) were measured for the
) were measured for the  Cs(n,
Cs(n, )
)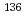 Cs reaction by an activation method and mass spectrometry. We used
Cs reaction by an activation method and mass spectrometry. We used  Cs contained as an impurity in a normally available
Cs contained as an impurity in a normally available  Cs standard solution. An isotope ratio of
Cs standard solution. An isotope ratio of  Cs and
Cs and  Cs in a standard
Cs in a standard  Cs solution was measured by mass spectrometry to quantify
Cs solution was measured by mass spectrometry to quantify  Cs. The analyzed
Cs. The analyzed  Cs samples were irradiated at the hydraulic conveyer of the research reactor in Institute for Integral Radiation and Nuclear Science, Kyoto University. Wires of Co/Al and Au/Al alloys were used as neutron monitors to measure thermal-neutron fluxes and epi-thermal Westcott's indices at an irradiation position. A gadolinium filter was used to measure the
Cs samples were irradiated at the hydraulic conveyer of the research reactor in Institute for Integral Radiation and Nuclear Science, Kyoto University. Wires of Co/Al and Au/Al alloys were used as neutron monitors to measure thermal-neutron fluxes and epi-thermal Westcott's indices at an irradiation position. A gadolinium filter was used to measure the 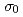 , and a value of 0.133 eV was taken as the cut-off energy. Gamma-ray spectroscopy was used to measure induced activities of
, and a value of 0.133 eV was taken as the cut-off energy. Gamma-ray spectroscopy was used to measure induced activities of  Cs,
Cs, 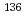 Cs and monitor wires. On the basis of Westcott's convention, the
Cs and monitor wires. On the basis of Westcott's convention, the 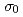 and I
and I values were derived as 8.57
values were derived as 8.57 0.25 barn, and 45.3
0.25 barn, and 45.3 3.2 barn, respectively. The
3.2 barn, respectively. The 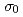 obtained in the present study agreed within the limits of uncertainties with the past reported value of 8.3
obtained in the present study agreed within the limits of uncertainties with the past reported value of 8.3 0.3 barn.
0.3 barn.
 Cs, and their time dependence
Cs, and their time dependenceWakiyama, Yoshifumi*; Onda, Yuichi*; Yoshimura, Kazuya; Igarashi, Yasunori*; Kato, Hiroaki*
Journal of Environmental Radioactivity, 210, p.105990_1 - 105990_12, 2019/12
Times Cited Count:22 Percentile:60.05(Environmental Sciences)Nakamura, Shoji; Kimura, Atsushi; Iwamoto, Osamu; Shibahara, Yuji*; Uehara, Akihiro*; Fujii, Toshiyuki*
KURNS Progress Report 2018, P. 106, 2019/08
Under the ImPACT project, the neutron capture cross-section measurements of Cesium-135 ( Cs) among the long-lived fission products have been performed at Kyoto University. This paper reports measurements of the thermal-neutron capture cross-section of
Cs) among the long-lived fission products have been performed at Kyoto University. This paper reports measurements of the thermal-neutron capture cross-section of  Cs at the Kyoto University Research Reactor (KUR).
Cs at the Kyoto University Research Reactor (KUR).
Malins, A.; Kurikami, Hiroshi; Kitamura, Akihiro; Machida, Masahiko
Remediation Measures for Radioactively Contaminated Areas, p.259 - 272, 2019/00