Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yuan, X.*; Hu, Q. H.*; Fang, X.*; Wang, Q. M.*; Ma, Y.*; Tachi, Yukio
Sedimentary Geology, 465, p.106633_1 - 106633_14, 2024/05
Times Cited Count:0 Percentile:0.00(Geology)Vauchy, R.; Hirooka, Shun; Matsumoto, Taku; Kato, Masato
Frontiers in Nuclear Engineering (Internet), 1, p.1060218_1 - 1060218_18, 2022/12
 and GeO
and GeO liquids
liquidsKawaguchi, Munemichi; Uno, Masayoshi*
Journal of Crystal Growth, 585, p.126590_1 - 126590_7, 2022/05
Phase-field mobility,  , and crystal growth rates in crystallization of 11 oxides or mixed oxides in undercooled silicates, SiO
, and crystal growth rates in crystallization of 11 oxides or mixed oxides in undercooled silicates, SiO and GeO
and GeO liquids were calculated with a simple phase-field model (PFM), and material dependence of the
liquids were calculated with a simple phase-field model (PFM), and material dependence of the  was discussed. Ratios between experimental crystal growth rates and the PFM simulation with
was discussed. Ratios between experimental crystal growth rates and the PFM simulation with 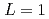 were confirmed to be proportional to a power of
were confirmed to be proportional to a power of 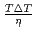 on the solid/liquid interface process during the crystal growth in a log-log plot. We determined that parameters,
on the solid/liquid interface process during the crystal growth in a log-log plot. We determined that parameters, 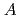 and
and  , of the
, of the 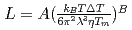 were
were 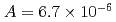 to
to 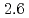 m
m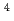 J
J s
s and
and 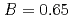 to
to 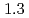 , which were unique for the materials. It was confirmed that our PFM simulation with the determined
, which were unique for the materials. It was confirmed that our PFM simulation with the determined  reproduced quantitively the experimental crystal growth rates. The
reproduced quantitively the experimental crystal growth rates. The 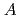 has a proportional relationship with the diffusion coefficient of a cation molar mass average per unit an oxygen molar mass at
has a proportional relationship with the diffusion coefficient of a cation molar mass average per unit an oxygen molar mass at 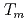 in a log-log graph. The
in a log-log graph. The  depends on the sum of the cation molar mass per the oxygen molar mass,
depends on the sum of the cation molar mass per the oxygen molar mass, 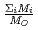 , in a compound. In
, in a compound. In 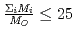 , the
, the  decreases with the cation molar mass increasing. The assumed cause is that the B represents the degree of the temperature dependence of the
decreases with the cation molar mass increasing. The assumed cause is that the B represents the degree of the temperature dependence of the  . Since the cation molar mass is proportional to an inertial resistance of the cation transfer, the
. Since the cation molar mass is proportional to an inertial resistance of the cation transfer, the  decreases with inverse of the cation molar mass. In crystallization of the silicates of heavy cation in
decreases with inverse of the cation molar mass. In crystallization of the silicates of heavy cation in 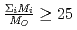 , the
, the  saturates at approximately 0.67, which leads to
saturates at approximately 0.67, which leads to 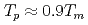 .
.
Hirooka, Shun; Yokoyama, Keisuke; Kato, Masato
Proceedings of International Conference on Fast Reactors and Related Fuel Cycles; Sustainable Clean Energy for the Future (FR22) (Internet), 8 Pages, 2022/04
Property studies on Am/Np-bearing MOX were carried out and how the properties influences on the irradiation behaviors was discussed. Both Am and Np inclusions increase the oxygen potential of MOX. Inter-diffusion coefficients obtained by using diffusion couple technique indicate that the inter-diffusion coefficient is larger in the order of U-Am, U-Pu and U-Np. Also, the inter-diffusion coefficients were evaluated to be larger at the O/M = 2 than those of O/M  2 by several orders. The increase of oxygen potential with Am/Np leads to higher vapor pressure of UO
2 by several orders. The increase of oxygen potential with Am/Np leads to higher vapor pressure of UO and the acceleration of the pore migration along temperature gradient during irradiation. The redistributions of actinide elements were also considered with the relationship of the pore migration and diffusion in solid state. Thus, the obtained inter-diffusion coefficients directly influence on the redistribution rate. The obtained properties were modelled and can be installed in a fuel irradiation simulation code.
and the acceleration of the pore migration along temperature gradient during irradiation. The redistributions of actinide elements were also considered with the relationship of the pore migration and diffusion in solid state. Thus, the obtained inter-diffusion coefficients directly influence on the redistribution rate. The obtained properties were modelled and can be installed in a fuel irradiation simulation code.
 -dispersed LATP by means of neutron radiography
-dispersed LATP by means of neutron radiographySong, F.*; Chen, H.*; Hayashida, Hirotoshi*; Kai, Tetsuya; Shinohara, Takenao; Yabutsuka, Takeshi*; Yao, Takeshi*; Takai, Shigeomi*
Solid State Ionics, 377, p.115873_1 - 115873_6, 2022/04
Times Cited Count:4 Percentile:29.55(Chemistry, Physical)Mukai, Masayuki; Ueda, Masato; Inada, Daisuke; Yukawa, Kazuhiko; Maeda, Toshikatsu; Iida, Yoshihisa
Proceedings of International Symposium NUCEF 2005, p.219 - 224, 2005/08
For better quantitative understanding of radionuclide migration for safety assessment of geologic disposal, JAERI has been conducting experimental and modeling studies on influences of humic substances, highly alkaline conditions and colloids on sorptive and diffusional behavior of TRU in geologic materials. In the absence of fulvic acid, one of humic substances, diffusion of Am through a tuff sample was not detected. By adding fulvic acid, Am was detected in the downstream cell, which indicates the diffusion through the sample. Highly alkaline conditions arisen from cementitious materials may spread by altering chemical and physical properties of geologic materials. Through-diffusion experiments of alkaline species in granite showed that the effective diffusion coefficient of Ca and OH
and OH in a cement-equilibrated aqueous solution were found to be higher by almost two orders of magnitude than Na
in a cement-equilibrated aqueous solution were found to be higher by almost two orders of magnitude than Na and OH
and OH in a NaOH solution. Radionuclide migration can be enhanced by colloids, and thus a calculation code describing the effect of colloids on radionuclide migration has been required.
in a NaOH solution. Radionuclide migration can be enhanced by colloids, and thus a calculation code describing the effect of colloids on radionuclide migration has been required.
 and UCl
and UCl dissolved in a LiCl-KCl eutectic melt
dissolved in a LiCl-KCl eutectic meltKuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Journal of the Electrochemical Society, 152(4), p.C203 - C212, 2005/04
Times Cited Count:117 Percentile:95.00(Electrochemistry)The electrochemical behavior of UCl and UCl
and UCl dissolved in LiCl-KCl eutectic melt was studied at 723-823 K. Electroreduction of U(IV) in LiCl-KCl melt occurs via two successive steps involving transfer of one and three electrons. The diffusion coefficients of U(IV) and U(III) ions were determined by linear sweep voltammetry, chronopotentiometry, and chronoamperometry. The formal standard potential of E
dissolved in LiCl-KCl eutectic melt was studied at 723-823 K. Electroreduction of U(IV) in LiCl-KCl melt occurs via two successive steps involving transfer of one and three electrons. The diffusion coefficients of U(IV) and U(III) ions were determined by linear sweep voltammetry, chronopotentiometry, and chronoamperometry. The formal standard potential of E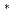 U(IV)/U(III), E
U(IV)/U(III), E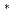 U(IV)/U, and E
U(IV)/U, and E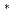 U(III)/U were determined and some thermodynamic properties of UCl
U(III)/U were determined and some thermodynamic properties of UCl and UCl
and UCl dissolved in LiCl-KCl eutectic melt were calculated. Influence of oxide ions on electrochemical behavior was also studied.
dissolved in LiCl-KCl eutectic melt were calculated. Influence of oxide ions on electrochemical behavior was also studied.
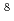 Li
LiJeong, S.-C.*; Katayama, Ichiro*; Kawakami, Hirokane*; Watanabe, Yutaka*; Ishiyama, Hironobu*; Miyatake, Hiroari*; Sataka, Masao; Okayasu, Satoru; Sugai, Hiroyuki; Ichikawa, Shinichi; et al.
Nuclear Instruments and Methods in Physics Research B, 230(1-4), p.596 - 600, 2005/04
Times Cited Count:6 Percentile:44.85(Instruments & Instrumentation)no abstracts in English
Jeong, S.-C.*; Katayama, Ichiro*; Kawakami, Hirokane*; Ishiyama, Hironobu*; Miyatake, Hiroari*; Sataka, Masao; Iwase, Akihiro*; Okayasu, Satoru; Sugai, Hiroyuki; Ichikawa, Shinichi; et al.
Japanese Journal of Applied Physics, Part 1, 42(7A), p.4576 - 4583, 2003/07
Times Cited Count:14 Percentile:49.69(Physics, Applied)no abstracts in English
Yamaguchi, Tetsuji; Nakayama, Shinichi
JAERI-Conf 2002-004, p.325 - 332, 2002/03
When long-lived radionuclides are transported by groundwater through fractures in the rock mass surrounding a radioactive waste repository, their diffusion into pores in the rock matrix and ensuing sorption onto mineral surfaces are expected to retard their transport along the pathways. We have characterized the pore structure of the Japanese Inada biotite granite and confirmed that Fick's diffusion law can be applied to the transport of aqueous species in granite. Effective diffusivity was determined by the through-diffusion method for cationic, anionic and actinide complex species to explore the mechanism of diffusion of the aqueous species. The results of this study enable us to provide a scientifically sound basis for radionuclide diffusion in granite for performance assessment of geological disposal. Future studies should emphasize understanding the diffusion mechanisms in low-permeability engineered barrier materials, data acquisition on long-term degradation of the materials and quantifying uncertainties associated with long-term mass transport analysis.
Fujimoto, Nozomu; Nojiri, Naoki; Takada, Eiji*; Saito, Kenji; Kobayashi, Shoichi; Sawahata, Hiroaki; Kokusen, Shigeru
JAERI-Tech 2000-091, 49 Pages, 2001/03
no abstracts in English
Kozai, Naofumi; Inada, Koichi*; Kozaki, Tamotsu*; Sato, Seichi*; Ohashi, Hiroshi*; Bamba, Tsunetaka
Journal of Contaminant Hydrology, 47(2-4), p.149 - 158, 2001/02
Times Cited Count:15 Percentile:41.05(Environmental Sciences)no abstracts in English
Minato, Kazuo; Ogawa, Toru; Sawa, Kazuhiro; Sekino, Hajime; Koya, Toshio; Kitagawa, Isamu; Ishikawa, Akiyoshi; Tomita, Takeshi;
Proc. of the Int. Conf. on Future Nuclear Systems (GLOBAL'99)(CD-ROM), 8 Pages, 1999/00
no abstracts in English
Akino, Fujiyoshi; Takeuchi, Motoyoshi; Ono, Toshihiko; Kaneko, Yoshihiko
Journal of Nuclear Science and Technology, 34(2), p.185 - 192, 1997/02
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)no abstracts in English
 C
CMinato, Kazuo; Ogawa, Toru; Fukuda, Kosaku; Sekino, Hajime; Kitagawa, Isamu; Mita, Naoaki
Journal of Nuclear Materials, 249(2-3), p.142 - 149, 1997/00
Times Cited Count:61 Percentile:95.79(Materials Science, Multidisciplinary)no abstracts in English
Kato, Shohei; Yanase, Yoshiaki; Honda, Tetsutaro*
IRPA9: 1996 International Congress on Radiation Protection, Proceedings, 3, p.354 - 356, 1996/00
no abstracts in English
 C
CMinato, Kazuo; Ogawa, Toru; Fukuda, Kosaku; H.Nabielek*; Sekino, Hajime; ;
Journal of Nuclear Materials, 224, p.85 - 92, 1995/00
Times Cited Count:60 Percentile:97.27(Materials Science, Multidisciplinary)no abstracts in English
J.Dellwo*; G.D.Alton*; J.C.Batchelder*; J.Breitenbach*; Carter, H. K.*; J.A.Chediak*; G.di-Bartolo*; ; Jentoff-Nilsen, K.*; J.Kormicki*
Nuclear Instruments and Methods in Physics Research B, 99, p.335 - 337, 1995/00
Times Cited Count:0 Percentile:0.00(Instruments & Instrumentation)no abstracts in English
Murata, Mikio; Noguchi, Hiroshi
Nihon Genshiryoku Gakkai-Shi, 34(2), p.149 - 152, 1992/02
Times Cited Count:2 Percentile:42.65(Nuclear Science & Technology)no abstracts in English
Sawa, Kazuhiro; Tazawa, Yujiro*; Itakura, Hirofumi*
JAERI-M 91-128, 33 Pages, 1991/08
no abstracts in English