Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
杉浦 佑樹; 石寺 孝充; 舘 幸男
Applied Clay Science, 200, p.105910_1 - 105910_10, 2021/01
被引用回数:14 パーセンタイル:75.75(Chemistry, Physical)In the geological disposal system, increase of Ca concentration with the alteration of cementitious materials would affect the retention of other radionuclides by competitive sorption. Batch sorption experiments were performed to investigate sorption behavior of Ca and competition with other divalent cations (Sr and Ni) on the edge site of montmorillonite under alkaline conditions. Ca and Sr formed surface complexation with the edge site at higher pH region compared to Ni. Sr sorption decreased with Ca concentration in alkaline pH region, whereas Ni sorption was not affected by Ca concentration. These results indicate that Ca and Sr sorb onto the same site while Ca and Ni sorb onto different sites, and competitive sorption depends on the chemical similarity such as hydrolysis behavior. Sorption model parameters obtained from the single element batch sorption experiments successfully reproduced the results of competitive sorption experiments.
杉浦 佑樹; 石寺 孝充; 舘 幸男
no journal, ,
We measured the sorption of Ca and its competitive sorption with Sr and Ni at high pH and ionic strength conditions. Sorption data were then evaluated using a thermodynamic sorption model. 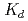 values of Ca increased with increasing pH values in alkaline pH region and the tendency was more pronounced at high ionic strength, indicating sorption onto edge sites. Fitting analyses show that Ca sorbs onto
values of Ca increased with increasing pH values in alkaline pH region and the tendency was more pronounced at high ionic strength, indicating sorption onto edge sites. Fitting analyses show that Ca sorbs onto  S
S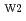 OH sites, with CaOH
OH sites, with CaOH sorption onto planar sites contributing at high alkaline pH regions. In competitive sorption experiments, Sr sorption onto the edge site decreased with increasing Ca concentration. In contrast, Ni sorption onto the edge site was independent of Ca. These results indicate that Sr sorbs onto the same edge sites (
sorption onto planar sites contributing at high alkaline pH regions. In competitive sorption experiments, Sr sorption onto the edge site decreased with increasing Ca concentration. In contrast, Ni sorption onto the edge site was independent of Ca. These results indicate that Sr sorbs onto the same edge sites ( S
S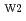 OH) as Ca, while Ni sorbs onto different edge sites (
OH) as Ca, while Ni sorbs onto different edge sites ( S
S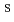 OH). Model calculations using the obtained sorption parameters reproduce the results of competitive sorption experiments.
OH). Model calculations using the obtained sorption parameters reproduce the results of competitive sorption experiments.