Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Zhao, Y.; Yoshimura, Kimio; Shishitani, Hideyuki*; Yamaguchi, Susumu*; Tanaka, Hirohisa*; Koizumi, Satoshi*; Szekely, N.*; Radulescu, A.*; Richter, D.*; Maekawa, Yasunari
Soft Matter, 12(5), p.1567 - 1578, 2016/02
Times Cited Count:30 Percentile:80.19(Chemistry, Physical)Chen, J.; Asano, Masaharu; Yamaki, Tetsuya; Yoshida, Masaru
Journal of Membrane Science, 269(1-2), p.194 - 204, 2006/02
Times Cited Count:141 Percentile:95.81(Engineering, Chemical)To develop a highly chemically stable polymer electrolyte membrane for application in a direct methanol fuel cell (DMFC), four styrene derivative monomers, m,p-methylstyrene (MeSt), p-tert-butylstyrene (tBuSt), divinylbenzene (DVB) and bis(p,p-vinyl phenyl) ethane (BVPE) were graft copolymerized into poly(ethylene-co-tetrafluoroethylene) (ETFE) films followed by sulfonation and hydrolysis. The latter two monomers were used as crosslinkers. The graft copolymerization was carried out by the  -ray preirradiation method. The influence of the preirradiation dose and the grafting kinetics were investigated in detail. Sulfonation of the grafted ETFE films was performed in a chlorosulfonic acid solution, by which the sulfonation ratio reached about 90%. The newly obtained membrane possesses significantly higher chemical stability than the traditional styrene/DVB-grafted membrane and six times lower methanol permeability compared to the Nafion 112 membrane. Therefore, this study reveals the possibility of the developed inexpensive four monomers-grafted membranes, which could provide an attractive alternative as a substitute for the expensive Nafion membranes for DMFC applications.
-ray preirradiation method. The influence of the preirradiation dose and the grafting kinetics were investigated in detail. Sulfonation of the grafted ETFE films was performed in a chlorosulfonic acid solution, by which the sulfonation ratio reached about 90%. The newly obtained membrane possesses significantly higher chemical stability than the traditional styrene/DVB-grafted membrane and six times lower methanol permeability compared to the Nafion 112 membrane. Therefore, this study reveals the possibility of the developed inexpensive four monomers-grafted membranes, which could provide an attractive alternative as a substitute for the expensive Nafion membranes for DMFC applications.
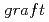 -poly(alkyl vinyl ether) membranes for polymer electrolyte membrane fuel cells by radiation processing
-poly(alkyl vinyl ether) membranes for polymer electrolyte membrane fuel cells by radiation processingChen, J.; Asano, Masaharu; Yamaki, Tetsuya; Yoshida, Masaru
Journal of Membrane Science, 256(1-2), p.38 - 45, 2005/06
New polymer electrolyte membranes having sulfonic acid groups for polymer electrolyte membrane fuel cell applications were prepared by simultaneous radiation-induced grafting method. The poly(tetrafluoroethylene) (PTFE) films, crosslinked by electron-beam radiation at molten temperature, were used as substrates for grafting of two alkyl vinyl ether monomers, propyl vinyl ether (nPVE) and isopropyl vinyl ether (iPVE), under controlled grafting conditions followed by sulfonation reactions. Thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), water contact angle and Fourier transform infrared (FTIR) were used to characterize the crosslinked PTFE (cPTFE) and grafted cPTFE films. The degree of grafting was found to be dependent on the grafting parameters such as irradiation temperature and Lewis acid catalyst, in which in the presence of Lewis acid catalyst or at a temperature close to the boiling point of each monomer, the grafting reaction significantly accelerated even when the relatively low dose was irradiated. Finally, the grafted cPTFE films were sulfonated in a chlorosulfonic acid solution. In spite of the lower ion-exchange capacity (0.75 mmol/g), the membrane synthesized in this study showed a proton conductivity as high as the Nafion 112.
Kavakli, P. A.*; Seko, Noriaki; Tamada, Masao; G ven, O.*
ven, O.*
Separation Science and Technology, 39(7), p.1631 - 1643, 2005/00
Times Cited Count:58 Percentile:83.54(Chemistry, Multidisciplinary)A new type of fibrous adsorbent with excess amidoxime groups was synthesized by radiation-induced graft polymerization. Glycidyl methacrylate (GMA) was first radiation-grafted on polyethylene-coated polypropylene nonwoven fabrics and chemically modified with 3,30-iminodipropionitrile [NH (-CH -CH
-CH -CN)
-CN) ] (IDPN), which was further reacted with hydroxylamine to obtain graft chains containing two amidoxime groups per graft repeating units. The adsorption properties of this new adsorbent for uranium (U), vanadium (V), lead (Pb), copper (Cu), and cobalt (Co) ions at low concentrations (3.3-1000 ppb).
] (IDPN), which was further reacted with hydroxylamine to obtain graft chains containing two amidoxime groups per graft repeating units. The adsorption properties of this new adsorbent for uranium (U), vanadium (V), lead (Pb), copper (Cu), and cobalt (Co) ions at low concentrations (3.3-1000 ppb).
Yamaki, Tetsuya; Kobayashi, Kazuhiro; Asano, Masaharu; Kubota, Hitoshi*; Yoshida, Masaru
Polymer, 45(19), p.6569 - 6573, 2004/09
Times Cited Count:56 Percentile:82.15(Polymer Science)We prepared proton exchange membranes by the  -ray-induced post grafting of styrene into crosslinked polytetrafluoroethylene (PTFE) films and subsequent sulfonation. The degree of grafting was controlled in the range of 7-75% by the crosslinking density of the PTFE matrix as well as the grafting conditions. Under our preparation conditions, the films at the grafting yield of
-ray-induced post grafting of styrene into crosslinked polytetrafluoroethylene (PTFE) films and subsequent sulfonation. The degree of grafting was controlled in the range of 7-75% by the crosslinking density of the PTFE matrix as well as the grafting conditions. Under our preparation conditions, the films at the grafting yield of  30% were found to produce ion exchange membranes with a homogeneous distribution of sulfonic acid groups. The resulting membranes showed a large ion exchange capacity up to 2.9 meq g
30% were found to produce ion exchange membranes with a homogeneous distribution of sulfonic acid groups. The resulting membranes showed a large ion exchange capacity up to 2.9 meq g , which exceeded the performance of commercially-available perfluorosulfonic acid films such as Nafion; nevertheless, they appeared to be dimensionally stable in water. These should undoubtedly result from the use of the crosslinked PTFE films as graft substrates and make our ion exchange membranes promising for applications to polymer electrolyte fuel cells.
, which exceeded the performance of commercially-available perfluorosulfonic acid films such as Nafion; nevertheless, they appeared to be dimensionally stable in water. These should undoubtedly result from the use of the crosslinked PTFE films as graft substrates and make our ion exchange membranes promising for applications to polymer electrolyte fuel cells.
Seko, Noriaki; Basuki, F.*; Tamada, Masao; Yoshii, Fumio
Reactive and Functional Polymers, 59(3), p.235 - 241, 2004/07
Times Cited Count:52 Percentile:81.16(Chemistry, Applied)Fibrous arsenic(As) adsorbent was synthesized by loading zirconium(Zr) on fibrous phosphoric adsorbent which was directly synthesized by radiation-induced grafting of 2-hydroxyethyl methacrylate phosphoric acid on polyethylene-coated polypropylene nonwoven fabric. Zirconium reacted with phosphoric acid grafted in the polyethylene layer. Zirconium density of the resulting adsorbent was 4.1 mmol/g. The breakthrough curve of As(V) adsorption was independent of the flow rate up to 1300 h in space velocity. The total capacity of As(V) was 2.0 mmol/ g-adsorbent at pH of 2. The adsorbed Zr(IV) could be evaluated by 0.4 M sodium hydroxide solution because no Zr(IV) could be found in the eluted solution. Anions of chloride and nitrate interfered the breakthrough capacity.
in space velocity. The total capacity of As(V) was 2.0 mmol/ g-adsorbent at pH of 2. The adsorbed Zr(IV) could be evaluated by 0.4 M sodium hydroxide solution because no Zr(IV) could be found in the eluted solution. Anions of chloride and nitrate interfered the breakthrough capacity.
; ; ; Hatada, Motoyoshi
Journal of Applied Polymer Science, 55, p.1643 - 1649, 1995/00
Times Cited Count:0 Percentile:0.00(Polymer Science)no abstracts in English
; ; ; ; Hatada, Motoyoshi
Journal of Applied Polymer Science, 51, p.841 - 853, 1994/00
Times Cited Count:11 Percentile:47.85(Polymer Science)no abstracts in English
 -ray irradiation
-ray irradiationG.M.Qin*; ; Hatada, Motoyoshi
JAERI-M 91-106, 114 Pages, 1991/07
no abstracts in English
JAERI-M 91-054, 44 Pages, 1991/03
no abstracts in English
;
Seni Gakkai-Shi, 45(7), p.318 - 323, 1989/07
no abstracts in English
;
Porifairu, 26(9), p.25 - 27, 1989/00
no abstracts in English
JAERI-M 84-239, 59 Pages, 1985/01
no abstracts in English
JAERI-M 83-199, 83 Pages, 1983/11
no abstracts in English
JAERI-M 82-192, 95 Pages, 1982/12
no abstracts in English
; ; ; ; ; Machi, Sueo
J.Appl.Polym.Sci., 27, p.1043 - 1051, 1982/00
Times Cited Count:92 Percentile:96.11(Polymer Science)no abstracts in English
; ; ; ; ; Machi, Sueo
J.Appl.Polym.Sci., 27, p.1033 - 1041, 1982/00
Times Cited Count:123 Percentile:97.53(Polymer Science)no abstracts in English
E.A.Hegazy*; ; A.M.Dessouki*; A.Rabie*;
J.Appl.Polym.Sci., 27, p.535 - 543, 1982/00
Times Cited Count:78 Percentile:94.85(Polymer Science)no abstracts in English
JAERI-M 9856, 131 Pages, 1981/12
no abstracts in English
E.A.Hegazy*; ; A.Rabie*; A.M.Dessouki*;
J.Appl.Polym.Sci., 26, p.3871 - 3883, 1981/00
Times Cited Count:56 Percentile:92.09(Polymer Science)no abstracts in English