Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Uchida, Shunsuke*; Sato, Tomonori; Morishima, Yusuke*; Hirose, Tatsuya*; Miyazawa, Takahiro*; Kakinuma, Nagao*; Sato, Yoshiyuki*; Usui, Naoshi*; Wada, Yoichi*
Proceedings of 12th International Conference on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors (CD-ROM), p.19 - 29, 2005/00
Static and dynamic responses of stainless steel specimens exposed to H O
O and O
and O in high temperature water were evaluated by analyzing ECP and FDCI (frequency dependent complex impedance). The oxide films on the specimens were characterized by multilateral surface analyses, e.g., LRS, SIMS, XPS and direct electric resistance measurement. As a result of evaluation, it was confirmed that (1) corrosive condition of BWR normal water chemistry (NWC) was simulated by 100 ppb H
in high temperature water were evaluated by analyzing ECP and FDCI (frequency dependent complex impedance). The oxide films on the specimens were characterized by multilateral surface analyses, e.g., LRS, SIMS, XPS and direct electric resistance measurement. As a result of evaluation, it was confirmed that (1) corrosive condition of BWR normal water chemistry (NWC) was simulated by 100 ppb H O
O without co-existing O
without co-existing O , while that of hydrogen water chemistry (HWC) was simulated by 10 ppb H
, while that of hydrogen water chemistry (HWC) was simulated by 10 ppb H O
O , (2) ECP under HWC was as high as that under NWC, while dissolution rate of oxide film under HWC was much lower than that under NWC, (3) combination effects of electric resistance and dissolution rate of oxide caused same level ECP for both NWC and HWC, and (4) distinct weight loss of the specimen exposed to 100 ppb H
, (2) ECP under HWC was as high as that under NWC, while dissolution rate of oxide film under HWC was much lower than that under NWC, (3) combination effects of electric resistance and dissolution rate of oxide caused same level ECP for both NWC and HWC, and (4) distinct weight loss of the specimen exposed to 100 ppb H O
O was observed.
was observed.
 O
OKameda, Yasuo*; Sasaki, Motoya*; Usuki, Takeshi*; Otomo, Toshiya*; Ito, Keiji*; Suzuya, Kentaro; Fukunaga, Toshiharu*
Journal of Neutron Research, 11(3), p.153 - 163, 2003/09
We describe results of time-of-flight (TOF) neutron diffraction of the liquid water null-H O in order to investigate the effect of both the scattering angle and the neutron flight path ratio to the observed self-scattering intensities. An empirical inelasticity correction procedure is proposed using the self-scattering intensity observed for the null-H
O in order to investigate the effect of both the scattering angle and the neutron flight path ratio to the observed self-scattering intensities. An empirical inelasticity correction procedure is proposed using the self-scattering intensity observed for the null-H O.
O.
 , and H
, and H O relevant to edge plasma impurities
O relevant to edge plasma impuritiesShirai, Toshizo; Tabata, Tatsuo*; Tawara, Hiroyuki*
Atomic Data and Nuclear Data Tables, 79(1), p.143 - 184, 2001/09
Times Cited Count:61 Percentile:92.07(Physics, Atomic, Molecular & Chemical)no abstracts in English
Sakurai, Tsutomu*; Yokoyama, Atsushi
Journal of Nuclear Science and Technology, 37(9), p.814 - 820, 2000/09
no abstracts in English
 O+SO
O+SO atmosphere
atmosphereKurata, Yuji; Suzuki, Tomio; Shimizu, Saburo
JAERI-Research 2000-011, p.56 - 0, 2000/03
no abstracts in English
Sekiguchi, Hiromi*; Sekiguchi, Tetsuhiro; Kitajima, Yoshinori*; Baba, Yuji
Photon Factory Activity Report 1998, Part B, P. 66, 1999/11
no abstracts in English
 D)+H
D)+H O reaction
O reactionKurosaki, Yuzuru*; Takayanagi, Toshiyuki
Journal of Physical Chemistry A, 103(3), p.436 - 442, 1999/00
Times Cited Count:20 Percentile:52.96(Chemistry, Physical)no abstracts in English
Oda, Chie;
JNC TN8400 98-001, 14 Pages, 1998/11
Solubilities of amorphous stannic oxide, SnO (am) in Na-ClO
(am) in Na-ClO -Cl-SO
-Cl-SO aqueous systems were measured to quantitatively investigate the influences of the ligands OH
aqueous systems were measured to quantitatively investigate the influences of the ligands OH , Cl
, Cl and SO
and SO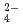 on solubilities. They were also measured in bentonite equilibrated solutions to discuss the behavior of tin under a repository condition of a high-revel radioactive waste. The solubility data in sodium perchlorate media in the range of pH from 6 to 11 showed pH dependency, and the hydrolysis constants of tin (IV) were determined (Amaya, et al., 1997). No significant changes in solubilities with the variation in Cl
on solubilities. They were also measured in bentonite equilibrated solutions to discuss the behavior of tin under a repository condition of a high-revel radioactive waste. The solubility data in sodium perchlorate media in the range of pH from 6 to 11 showed pH dependency, and the hydrolysis constants of tin (IV) were determined (Amaya, et al., 1997). No significant changes in solubilities with the variation in Cl , SO
, SO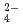 concentrations were observed in Na-ClO
concentrations were observed in Na-ClO -Cl-SO
-Cl-SO aqueous systems, so this indicates that chloride and sulfate species were less effective than hydroxide complexes. On the other hand, solubilities in bentonite equilibrated solutions were higher than solubilities of other experiments in simple systems. These results suggest that (1) other complexes of tin except hydroxide, chloride or sulfate complexes of tin (IV) may dominantly exist in aqueous phase, (2)solid phase other than SnO
aqueous systems, so this indicates that chloride and sulfate species were less effective than hydroxide complexes. On the other hand, solubilities in bentonite equilibrated solutions were higher than solubilities of other experiments in simple systems. These results suggest that (1) other complexes of tin except hydroxide, chloride or sulfate complexes of tin (IV) may dominantly exist in aqueous phase, (2)solid phase other than SnO (am) may limit the solubility of tin under repository conditions.
(am) may limit the solubility of tin under repository conditions.
Kimura, Takaumi; ; Yoshida, Zenko; Shirasu, Noriko
Journal of Nuclear Science and Technology, 33(6), p.519 - 521, 1996/06
no abstracts in English
 O/Si(100) adsorption system by O 1s excitation
O/Si(100) adsorption system by O 1s excitationSekiguchi, Tetsuhiro; Ikeura, Hiromi*; Tanaka, Kenichiro*; Obi, Kinichi*
Journal of Electron Spectroscopy and Related Phenomena, 80, p.65 - 68, 1996/00
Times Cited Count:4 Percentile:25.79(Spectroscopy)no abstracts in English
 partial pressures
partial pressures; Kimura, Takaumi; Yoshida, Zenko;
Radiochimica Acta, 74, p.21 - 25, 1996/00
no abstracts in English
Kimura, Takaumi; Choppin, G. R.*
Journal of Alloys and Compounds, 213-214, p.313 - 317, 1994/00
Times Cited Count:273 Percentile:99.70(Chemistry, Physical)no abstracts in English
 ions desorbed from H
ions desorbed from H O/Si(100) adsorption system by O 1s excitation
O/Si(100) adsorption system by O 1s excitationSekiguchi, Tetsuhiro; Ikeura, Hiromi*; Obi, Kinichi*; Tanaka, Kenichiro*
Photon Factory Activity Report, P. 128, 1994/00
no abstracts in English
 in carbonate solutions
in carbonate solutionsG.Meinrath*; Takeishi, Hideyo
Journal of Alloys and Compounds, 194, p.93 - 99, 1993/00
Times Cited Count:23 Percentile:82.40(Chemistry, Physical)no abstracts in English
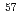 Fe-atoms after EC-decay of
Fe-atoms after EC-decay of 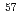 Co-labelled Co(BrO
Co-labelled Co(BrO )
)
 6H
6H O studied by coincidence Moessbauer spectroscopy
O studied by coincidence Moessbauer spectroscopyNakada, Masami; ; Nakahara, Hiromichi*;
Hyperfine Interactions, 70, p.1241 - 1244, 1992/00
Times Cited Count:2 Percentile:17.50(Physics, Atomic, Molecular & Chemical)no abstracts in English
; ; ; ;
Tanso, 116, p.2 - 9, 1984/00
no abstracts in English
 SO
SO -HNO
-HNO and H
and H SO
SO -H
-H O
O
; ; ; ;
Nucl.Chem.Waste Manage., 4, p.307 - 312, 1983/00
no abstracts in English
; ; ;
JAERI-M 82-087, 19 Pages, 1982/07
no abstracts in English
Ikezoe, Yasumasa; ; Shimizu, Saburo; Nakajima, Hayato
Int.J.Hydrogen Energy, 7(7), p.539 - 543, 1982/00
Times Cited Count:6 Percentile:73.82(Chemistry, Physical)no abstracts in English
Ikezoe, Yasumasa; Shimizu, Saburo; ; ; ;
Radiation Physics and Chemistry, 20(4), p.253 - 257, 1982/00
no abstracts in English