Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
伊藤 孝; 髭本 亘; 下村 浩一郎*
Physical Review B, 108(22), p.224301_1 - 224301_11, 2023/12
被引用回数:6 パーセンタイル:58.52(Materials Science, Multidisciplinary)In positive muon spin rotation and relaxation (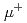 SR) spectroscopy, positive muons (
SR) spectroscopy, positive muons (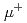 ) implanted into solid oxides are conventionally treated as immobile spin-probes at interstitial sites below room temperature. This is because each
) implanted into solid oxides are conventionally treated as immobile spin-probes at interstitial sites below room temperature. This is because each 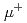 is thought to be tightly bound to an oxygen atom in the host lattice to form a muonic analogue of the hydroxy group. On the basis of this concept, anomalies in
is thought to be tightly bound to an oxygen atom in the host lattice to form a muonic analogue of the hydroxy group. On the basis of this concept, anomalies in 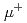 SR spectra observed in oxides have been attributed in most cases to the intrinsic properties of host materials. On the other hand, global
SR spectra observed in oxides have been attributed in most cases to the intrinsic properties of host materials. On the other hand, global 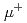 diffusion with an activation energy of
diffusion with an activation energy of  0.1~eV has been reported in some chemically-substituted perovskite oxides at cryogenic temperatures, although the reason for the small activation energy despite the formation of the strong O
0.1~eV has been reported in some chemically-substituted perovskite oxides at cryogenic temperatures, although the reason for the small activation energy despite the formation of the strong O bond has not yet been quantitatively understood. In this study, we investigated interstitial
bond has not yet been quantitatively understood. In this study, we investigated interstitial 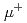 diffusion in the perovskite oxide lattice using KTaO
diffusion in the perovskite oxide lattice using KTaO cubic perovskite as a model system. We used the
cubic perovskite as a model system. We used the 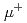 SR method and density functional theory calculations along with the harmonic transition state theory to study this phenomenon both experimentally and theoretically. Experimental activation energies for global
SR method and density functional theory calculations along with the harmonic transition state theory to study this phenomenon both experimentally and theoretically. Experimental activation energies for global 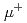 diffusion obtained below room temperature were less than a quarter of the calculated classical potential barrier height for a bottleneck
diffusion obtained below room temperature were less than a quarter of the calculated classical potential barrier height for a bottleneck 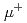 transfer path. The reduction in the effective barrier height could be explained by the harmonic transition state theory with a zero-point energy correction; a significant difference in zero-point energies for
transfer path. The reduction in the effective barrier height could be explained by the harmonic transition state theory with a zero-point energy correction; a significant difference in zero-point energies for 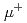 at the positions in the O
at the positions in the O bonding equilibrium state and a bond-breaking transition state was the primary cause of the reduction. This suggests that the assumption of immobile
bonding equilibrium state and a bond-breaking transition state was the primary cause of the reduction. This suggests that the assumption of immobile 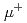 in solid oxides is not always satisfied since such a significant decrease in diffusion barrier height can also occur in other oxides.
in solid oxides is not always satisfied since such a significant decrease in diffusion barrier height can also occur in other oxides.