Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 hydridosilicate at high pressures; A Bridge to BaSiH
hydridosilicate at high pressures; A Bridge to BaSiH polyhydride
polyhydrideBeyer, D. C.*; Spektor, K.*; Vekilova, O. Y.*; Grins, J.*; Barros Brant Carvalho, P. H.*; Leinbach, L. J.*; Sannemo-Targama, M.*; Bhat, S.*; Baran, V.*; Etter, M.*; et al.
ACS Omega (Internet), 10(15), p.15029 - 15035, 2025/04
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)Hydridosilicates featuring SiH octahedral moieties represent a rather new class of compounds with potential properties relating to hydrogen storage and hydride ion conductivity. Here, we report on the new representative BaSiH
octahedral moieties represent a rather new class of compounds with potential properties relating to hydrogen storage and hydride ion conductivity. Here, we report on the new representative BaSiH obtained from reacting the Zintl phase hydride BaSiH
obtained from reacting the Zintl phase hydride BaSiH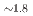 with H
with H fluid at pressures above 4 GPa and subsequent decompression to ambient pressure. It consists of complex SiH
fluid at pressures above 4 GPa and subsequent decompression to ambient pressure. It consists of complex SiH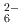 ions, which are octahedrally coordinated by Ba
ions, which are octahedrally coordinated by Ba counterions. The arrangement of Ba and Si atoms deviates only slightly from an ideal fcc NaCl structure. IR and Raman spectroscopy showed SiH
counterions. The arrangement of Ba and Si atoms deviates only slightly from an ideal fcc NaCl structure. IR and Raman spectroscopy showed SiH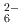 bending and stretching modes in the ranges 800-1200 and 1400-1800 cm
bending and stretching modes in the ranges 800-1200 and 1400-1800 cm , respectively. BaSiH
, respectively. BaSiH is thermally stable up to 95
is thermally stable up to 95 C above which decomposition into BaH
C above which decomposition into BaH and Si takes place. DFT calculations indicated a direct band gap of 2.5 eV. The discovery of BaSiH
and Si takes place. DFT calculations indicated a direct band gap of 2.5 eV. The discovery of BaSiH consolidates the compound class of hydridosilicates, accessible from hydrogenations of silicides at gigapascal pressures (
consolidates the compound class of hydridosilicates, accessible from hydrogenations of silicides at gigapascal pressures (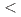 10 GPa). The structural properties of BaSiH
10 GPa). The structural properties of BaSiH suggest that it presents an intermediate (or precursor) for further hydrogenation at considerably higher pressures to the predicted superconducting polyhydride BaSiH
suggest that it presents an intermediate (or precursor) for further hydrogenation at considerably higher pressures to the predicted superconducting polyhydride BaSiH .
.
 inferred from muon study
inferred from muon studyKadono, Ryosuke*; Hiraishi, Masatoshi*; Okabe, Hirotaka*; Koda, Akihiro*; Ito, Takashi
Journal of Physics; Condensed Matter, 35(28), p.285503_1 - 285503_13, 2023/07
Times Cited Count:1 Percentile:11.20(Physics, Condensed Matter)Kawaguchi, Munemichi
Journal of Physical Chemistry C, 125(22), p.11813 - 11819, 2021/06
Times Cited Count:5 Percentile:24.25(Chemistry, Physical)Isothermal and constant heating thermogravimetry-differential thermal analysis (TG-DTA) and Fourier transform infrared spectrometer (FTIR) measurements have been performed for pre- and post-fired sodium hydride (NaH) in the temperature range of 500-700 K, respectively. Temperature dependence of NaH thermal decomposition rates obtained by the isothermal TGs showed an inflection point at around 620 K, which was caused by two kinds of hydrogen states (rapid diffusing and immobile hydrogen). In the FTIR spectra for the NaH and sodium (Na), the specific signals were observed at around 873.4, 1010.4, 1049.5 and 1125.7 cm , and the integrated values of FTIR signals for post-fired NaH at below 550K and at above 698 K were comparable to those for pre-fired NaH and Na, respectively. Those for post-fired NaH at 602-667 K were the intermediate values of the pre-fired NaH and Na, which denoted that the Na-Na bonds haven't grown sufficiently and the hydrogen coexisted in metallic Na. In order to predict the practical kinetics of NaH thermal decomposition reaction, we suggested the simple kinetics model which assumed two kinds of rapidly diffusing and immobile hydrogen states. The simulation results revealed the inflection point in temperature dependence of the thermal decomposition rates accordingly because the transition from immobile hydrogen to rapid diffusing hydrogen crosses over at around 620 K.
, and the integrated values of FTIR signals for post-fired NaH at below 550K and at above 698 K were comparable to those for pre-fired NaH and Na, respectively. Those for post-fired NaH at 602-667 K were the intermediate values of the pre-fired NaH and Na, which denoted that the Na-Na bonds haven't grown sufficiently and the hydrogen coexisted in metallic Na. In order to predict the practical kinetics of NaH thermal decomposition reaction, we suggested the simple kinetics model which assumed two kinds of rapidly diffusing and immobile hydrogen states. The simulation results revealed the inflection point in temperature dependence of the thermal decomposition rates accordingly because the transition from immobile hydrogen to rapid diffusing hydrogen crosses over at around 620 K.
Abe, Hiroshi; Tokuhira, Shinnosuke*; Uchida, Hirohisa*; Oshima, Takeshi
Nuclear Instruments and Methods in Physics Research B, 365(Part A), p.214 - 217, 2015/12
no abstracts in English
 study of hydrogen hydrate clathrates for hydrogen storage within the ITBL environment
study of hydrogen hydrate clathrates for hydrogen storage within the ITBL environmentSluiter, M. H. F.*; Belosludov, R. V.*; Jain, A.*; Belosludov, V. R.*; Adachi, Hitoshi*; Kawazoe, Yoshiyuki*; Higuchi, Kenji; Otani, Takayuki
Lecture Notes in Computer Science 2858, p.330 - 341, 2003/00
Recently, for the first time a hydrate clathrate was discovered with hydrogen. Aside from the great technological promise that is inherent in storing hydorogen at high density at modest pressures, there is great scientefic interest as this would constitute the first hydrate clathrate with multiple guest molecules per cage. The multiple cage occupancy is controversial, and reproducibility of the experiments has been questioned. Therefore in this study we try to illucidate the remarkable stability of the hydrogen hydrate clathrate, and determine the thermodynamically most favored cage occpancy using highly accrate  computer simulations in a parameter survey. To carry out these extraordinary demanding computations a distributed
computer simulations in a parameter survey. To carry out these extraordinary demanding computations a distributed  code has been developed using the SuperSINET with the ITBL software as the top-layer.
code has been developed using the SuperSINET with the ITBL software as the top-layer.