Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Arima, Tatsumi*; Idemitsu, Kazuya*; Inagaki, Yaohiro*; Kawamura, Katsuyuki*; Tachi, Yukio; Yotsuji, Kenji
Progress in Nuclear Energy, 92, p.286 - 297, 2016/09
Times Cited Count:15 Percentile:77.12(Nuclear Science & Technology)Diffusion and adsorption behavior of uranyl (UO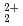 ) species is important for the performance assessment of radioactive waste disposal. The diffusion behaviors of UO
) species is important for the performance assessment of radioactive waste disposal. The diffusion behaviors of UO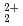 , K
, K , CO
, CO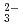 and Cl
and Cl and H
and H O in the aqueous solutions were evaluated by molecular dynamics (MD) calculations. The diffusion coefficient (De) of UO
O in the aqueous solutions were evaluated by molecular dynamics (MD) calculations. The diffusion coefficient (De) of UO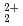 is the smallest and is 26% less than the self-diffusion coefficient of H
is the smallest and is 26% less than the self-diffusion coefficient of H O. For the aqueous solution with high concentration of carbonate ions, uranyl carbonate complexes: UO
O. For the aqueous solution with high concentration of carbonate ions, uranyl carbonate complexes: UO CO
CO and UO
and UO (CO
(CO )
) can be observed. For the clay (montmorillonite or illite)-aqueous solution systems, the adsorption and diffusion behaviors of UO
can be observed. For the clay (montmorillonite or illite)-aqueous solution systems, the adsorption and diffusion behaviors of UO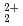 and K
and K were evaluated by MD calculations. The distribution coefficients (Kd) increase with the layer charge of clay, and Kd of UO
were evaluated by MD calculations. The distribution coefficients (Kd) increase with the layer charge of clay, and Kd of UO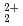 might be smaller than that of K
might be smaller than that of K . Further, their two-dimensional diffusion coefficients were relatively small in the adsorption layer and were extremely small for illite with higher layer charge.
. Further, their two-dimensional diffusion coefficients were relatively small in the adsorption layer and were extremely small for illite with higher layer charge.
Savage, D.*; Wilson, J.*; Benbow, S.*; Sasamoto, Hiroshi; Oda, Chie; Walker, C.*; Kawama, Daisuke*; Tachi, Yukio
no journal, ,
Safety functions for the clay buffer in a repository for HLW are fulfilled if the presence of montmorillonite is maintained in the long-term. Its transformation to non-swelling minerals (e.g. illite) is addressed in most safety assessments by using semi-empirical kinetic models. However, this approach contrasts with all other near-field geochemical modelling activities that employ complex reaction-transport simulations. Here we investigate the consistency of these two approaches by modelling the montmorillonite to illite transformation in the marine sediment profile penetrated by the Ocean Drilling Program (ODP) Site 1174. Illitisation of smectite at Site 1174 using the semi-empirical approach has been modeled by previous studies, and shown to provide a reasonable match to the gradual change of illite content with depth. In comparison, the initial results of reaction-transport simulations showed rapid (conservative) conversion of montmorillonite to illite. The cause of this rapid conversion appears to be the transformation of amorphous silica to quartz over a similar timescale. Subsequent simulations have focused on alternative mechanisms for mineral growth that may explain the discrepancies between the semi-empirical and reaction-transport approaches.
Wilson, J.*; Bateman, K.*; Kawama, Daisuke*; Tachi, Yukio
no journal, ,