Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
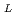 -proline methyl ester) gel membrane in LiCl solution
-proline methyl ester) gel membrane in LiCl solutionChen, J.; Asano, Masaharu; Tsubokawa, Norio*; Maekawa, Yasunari; Yamaki, Tetsuya; Yoshida, Masaru
Journal of Polymer Science, Part B; Polymer Physics, 43(20), p.2843 - 2851, 2005/10
Times Cited Count:0 Percentile:0.00(Polymer Science)Impedance spectra analysis of a thermo-responsive poly(acryloyl-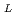 -proline methyl ester) (poly(A-ProOMe)) hydrogel membranes in an aqueous solution of LiCl was carried out using a simple equivalent model. The hydrogel membrane was synthesized by
-proline methyl ester) (poly(A-ProOMe)) hydrogel membranes in an aqueous solution of LiCl was carried out using a simple equivalent model. The hydrogel membrane was synthesized by  -radiation-induced polymerization and crosslinking of A-ProOMe monomer aqueous solution in a glass-cast. By means of the impedance spectra analysis, a novel method for the calculation of the ionic conductivity of the hydrogel membranes in LiCl solution was proposed. The calculated ionic conductivity was in agreement well with the determined value. In addition, effects of temperature and LiCl concentration on the impedance spectra and ionic conductivity of the gel membrane were analysized. Results indicated that the impedance spectra analysis is a very useful tool for evaluating the electric properties of gel membranes in an electrolyte solution. The poly(A-ProOMe) gel membrane in 1.0 M LiCl solution showed a high ionic conductivity of about 0.2 S/cm at 14
-radiation-induced polymerization and crosslinking of A-ProOMe monomer aqueous solution in a glass-cast. By means of the impedance spectra analysis, a novel method for the calculation of the ionic conductivity of the hydrogel membranes in LiCl solution was proposed. The calculated ionic conductivity was in agreement well with the determined value. In addition, effects of temperature and LiCl concentration on the impedance spectra and ionic conductivity of the gel membrane were analysized. Results indicated that the impedance spectra analysis is a very useful tool for evaluating the electric properties of gel membranes in an electrolyte solution. The poly(A-ProOMe) gel membrane in 1.0 M LiCl solution showed a high ionic conductivity of about 0.2 S/cm at 14 C. The temperature-dependence of the ionic conductivity was a complex nonlinear form due to the volume phase transition of the thermo-responsive poly(A-ProOMe) gel membrane, and the volume phase transition temperature appeared to be decreased with the increase in the LiCl concentration.
C. The temperature-dependence of the ionic conductivity was a complex nonlinear form due to the volume phase transition of the thermo-responsive poly(A-ProOMe) gel membrane, and the volume phase transition temperature appeared to be decreased with the increase in the LiCl concentration.
Nakamura, Akio; J. B. Wagner Jr.*
Proceedings of the Zirconia '86 Tokyo II, p.171 - 192, 1989/00
no abstracts in English
; Konishi, Satoshi; ; Kurasawa, T.; Katsuta, Hiroji; ;
Journal of Nuclear Materials, 132, p.222 - 230, 1985/00
Times Cited Count:18 Percentile:86.74(Materials Science, Multidisciplinary)no abstracts in English
 SiO
SiO and
and  -LiAlO
-LiAlO
; Konishi, Satoshi
J.Am.Ceram.Soc., 67(6), p.418 - 419, 1984/00
Times Cited Count:32 Percentile:84.88(Materials Science, Ceramics)no abstracts in English
; Furukawa, Kazuo
First Inter.Symp.on Molten Salt Chem.Technol.,J-315, p.449 - 452, 1983/00
no abstracts in English
Konishi, Satoshi; ; ; Naruse, Yuji
Nucl.Technol./Fusion, 3, p.195 - 198, 1983/00
no abstracts in English
 O,Li
O,Li SiO
SiO and LiAlO
and LiAlO
; Konishi, Satoshi; ; ; Katsuta, Hiroji; ; ;
JAERI-M 82-146, 33 Pages, 1982/10
no abstracts in English
 -methylstyrene
-methylstyrene; ;
Polym.J., 3(6), p.762 - 763, 1972/06
Times Cited Count:6no abstracts in English