Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -isosaccharinic acid
-isosaccharinic acidKobayashi, Taishi*; Sasaki, Takayuki*; Kitamura, Akira
Journal of Chemical Thermodynamics, 138, p.151 - 158, 2019/11
Times Cited Count:6 Percentile:23.95(Thermodynamics)The effect of  -isosaccharinic acid (ISA) on the solubility and redox of tetravalent and hexavalent uranium (U(IV), U(VI)) was investigated in the hydrogen ion concentration (pH
-isosaccharinic acid (ISA) on the solubility and redox of tetravalent and hexavalent uranium (U(IV), U(VI)) was investigated in the hydrogen ion concentration (pH ) range of 6
) range of 6 13 and at total ISA concentration ([ISA]
13 and at total ISA concentration ([ISA]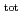 ) = 10
) = 10
 10
10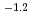 mol/dm
mol/dm . The dependence of U(IV) solubility on pH
. The dependence of U(IV) solubility on pH and [ISA]
and [ISA]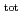 suggested the existence of U(OH)
suggested the existence of U(OH) (ISA)
(ISA)
 as a dominant species within the investigated pH
as a dominant species within the investigated pH range of 6
range of 6 12. For the U(VI)-ISA system, UO
12. For the U(VI)-ISA system, UO (OH)
(OH) (ISA)
(ISA)
 was suggested as a dominant species at pH
was suggested as a dominant species at pH 7
7 13. The formation constants of the U(IV)-ISA and U(VI)-ISA complexes were determined by least-squares fitting of the solubility data. The solubility of U(IV) and U(VI) in the presence of ISA and its effect on the redox behavior were thermodynamically interpreted based on the obtained constants.
13. The formation constants of the U(IV)-ISA and U(VI)-ISA complexes were determined by least-squares fitting of the solubility data. The solubility of U(IV) and U(VI) in the presence of ISA and its effect on the redox behavior were thermodynamically interpreted based on the obtained constants.
 -UO
-UO
 -CO
-CO
 system and integration with JAEA's thermodynamic database for geochemical calculations
system and integration with JAEA's thermodynamic database for geochemical calculationsKitamura, Akira
JAEA-Data/Code 2018-018, 103 Pages, 2019/03
The latest available thermodynamic data were critically reviewed and the selected values were included into the JAEA-TDB for performance assessment of geological disposal of high-level radioactive and TRU wastes. This critical review specifically addressed thermodynamic data for (1) a zirconium-hydroxide system through comparison of thermodynamic data selected by the Nuclear Energy Agency within the Organisation for Economic Co-operation and Development (OECD/NEA), (2) complexation of metal ions with isosaccharinic acid based on the latest review papers. Furthermore, the author performed (3) tentative selection of thermodynamic data on ternary complexes among alkaline-earth metal, uranyl and carbonate ions, and (4) integration with the latest version of JAEA's thermodynamic database for geochemical calculations. The internal consistency of the selected data was checked by the author. Text files of the updated and integrated thermodynamic database have been prepared for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
Rai, D.*; Kitamura, Akira
Journal of Chemical Thermodynamics, 114, p.135 - 143, 2017/11
Times Cited Count:12 Percentile:20.78(Thermodynamics)Isosaccharinic acid is a cellulose degradation product that can form in low-level nuclear waste repositories and is known to form strong complexes with many elements, including actinides, disposed of in these repositories. We (1) reviewed the available data for deprotonation and lactonisation constants of isosaccharinic acid, and the isosaccharinate binding constants for Ca, Fe(III), Th, U(IV), U(VI), Np(IV), Pu(IV), and Am(III), (2) summarized complexation constant values for predicting actinide behavior in geologic repositories in the presence of isosaccharinate, and (3) outlined additional studies to acquire reliable thermodynamic data where the available data are inadequate.
Kobayashi, Taishi*; Teshima, Takeshi*; Sasaki, Takayuki*; Kitamura, Akira
Journal of Nuclear Science and Technology, 54(2), p.233 - 241, 2017/02
Times Cited Count:9 Percentile:60.50(Nuclear Science & Technology)Zr solubility in the presence of gluconic acid (GLU) and isosaccharinic acid (ISA) was investigated as a function of hydrogen ion concentration (pH ) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH
) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH and GLU concentration suggested the existence of Zr(OH)
and GLU concentration suggested the existence of Zr(OH) (GLU)
(GLU)
 in the neutral pH region and Zr(OH)
in the neutral pH region and Zr(OH) (GLU)(GLU
(GLU)(GLU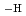 )
) in the alkaline pH region above pH
in the alkaline pH region above pH 10 as the dominant species in the presence of 10
10 as the dominant species in the presence of 10 - 10
- 10 mol/dm
mol/dm (M) GLU. In the presence of ISA, the dominant species Zr(OH)
(M) GLU. In the presence of ISA, the dominant species Zr(OH) (ISA)
(ISA)
 and Zr(OH)
and Zr(OH) (ISA)(ISA
(ISA)(ISA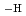 )
) were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH)
were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH) (am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.
(am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.